
(14��) �ں�����ߺ�DZˮͧ�п��ù�������������������ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ������ͼ�е�ʵ��װ�ý���ʵ�飬֤���������ƿ�����������?
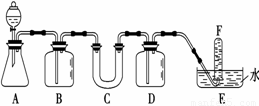
��1��A��ʵ������ȡCO2��װ�ã�д�����������������̼��Ӧ�Ļ�ѧ����ʽ��____________________��
��2����д���пո�
|
���� |
�����Լ� |
������Լ���Ŀ�� |
|
B |
����NaHCO3��Һ |
|
|
C |
|
|
|
D |
|
|
��3���Թ�F���ռ����������һ��ʵ������ǣ�������������������������������������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��) �ں�����ߺ�DZˮͧ�п��ù�������������������ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ������ͼ�е�ʵ��װ�ý���ʵ�飬֤���������ƿ�����������?
��1��A��ʵ������ȡCO2��װ�ã�д�����������������̼��Ӧ�Ļ�ѧ����ʽ��____________________��
��2����д���пո�
| ���� | �����Լ� | ������Լ���Ŀ�� |
| B | ����NaHCO3��Һ |
|
| C |
|
|
| D |
|
|
��3���Թ�F���ռ����������һ��ʵ������ǣ�������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��) �ں�����ߺ�DZˮͧ�п��ù�����������������ij����С�����������ʵ��װ�ã���֤���������ƿ�����������װ��A��B��C��ʢװ���Լ����ɹ�ѡ���Ϊ������NaHCO3��Һ��CaCO3���塢NaOH��Һ��1.95 g Na2O2���塣������װ�ûش����⣺

��1��A�з�����Ӧ�����ӷ���ʽΪ ��
��2�� Bƿ�������� ����Ӧ�����ӷ���ʽ ��
��3����D�е�ʯ��ˮ���ֳ�������ɫ���ǣ���˵��ԭ�� ___________________________________________________________________________��
��4����Ӧ���ʱ�������E�еļ���ƿ�ռ���������Ϊ250 mL����֪�������ܶ�Ϊ1.43g/L����װ�õ����������õ�����£�ʵ���ռ�����������������ۼ���ֵ ������С�������Լ mL��ȡ����ֵ���������ݾ��ڱ�״���²ⶨ������������ ____________________________________________��
��5��Ϊ��ʹװ��D���ָ���ȫ���������ɽ�װ��D��Ϊ���� װ�ã��üס��ҡ����ش�
6��ʵ����ϣ����ⶨC�й������ﺬNa2CO3������������ʵ�鲽�����£��������ʵ�鲽���еĿհס�
����ȡa g C�й�������һ������ˮ�У���������BaCl2��Һ�����ˣ� ��
��������b g���壬��Na2CO3����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ����ʮ���и����ڶ����¿���ѧ�Ծ� ���ͣ�ʵ����
(14��) �ں�����ߺ�DZˮͧ�п��ù�������������������ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ������ͼ�е�ʵ��װ�ý���ʵ�飬֤���������ƿ�����������?
��1��A��ʵ������ȡCO2��װ�ã�д�����������������̼��Ӧ�Ļ�ѧ����ʽ��____________________��
��2����д���пո�
| ���� | �����Լ� | ������Լ���Ŀ�� |
| B | ����NaHCO3��Һ | |
| C | | |
| D | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ����������һ�и�һ12���¿���ѧ�Ծ� ���ͣ�ʵ����
(15��) �ں�����ߺ�DZˮͧ�п��ù�����������������ij����С�����������ʵ��װ�ã���֤���������ƿ�����������װ��A��B��C��ʢװ���Լ����ɹ�ѡ���Ϊ������NaHCO3��Һ��CaCO3���塢NaOH��Һ��1.95 g Na2O2���塣 ������װ�ûش����⣺


��1��A�з�����Ӧ�����ӷ���ʽΪ ��
��2�� Bƿ�������� ����Ӧ�����ӷ���ʽ ��
��3����D�е�ʯ��ˮ���ֳ�������ɫ���ǣ���˵��ԭ�� ___________________________________________________________________________��
��4����Ӧ���ʱ�������E�еļ���ƿ�ռ���������Ϊ250 mL����֪�������ܶ�Ϊ1.43g/L����װ�õ����������õ�����£�ʵ���ռ�����������������ۼ���ֵ ������С�������Լ mL��ȡ����ֵ���������ݾ��ڱ�״���²ⶨ������������ ____________________________________________��

��5��Ϊ��ʹװ��D���ָ���ȫ���������ɽ�װ��D��Ϊ���� װ�ã��üס��ҡ����ش�
6��ʵ����ϣ����ⶨC�й������ﺬNa2CO3������������ʵ�鲽�����£��������ʵ�鲽���еĿհס�
����ȡa g C�й�������һ������ˮ�У���������BaCl2��Һ�����ˣ� ��
��������b g���壬��Na2CO3����������Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com