

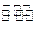 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��  �� Cu��2�֣�
�� Cu��2�֣� + NH4+ + 2Ag��+ 3NH3 + H2O��ˮԡ���ȣ���3�֣�
+ NH4+ + 2Ag��+ 3NH3 + H2O��ˮԡ���ȣ���3�֣� + 3SO2 + 6 H2O =" 2Au" + 8Cl
+ 3SO2 + 6 H2O =" 2Au" + 8Cl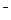 + 3SO42
+ 3SO42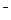 + 12H+��3�֣�
+ 12H+��3�֣�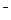 ��Au3+��Cl��������AuCl4
��Au3+��Cl��������AuCl4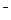 ��ʹ��ƽ����Au3+Ũ�Ƚ��ͣ�ƽ�����ƣ���������ˮ����3�֣�
��ʹ��ƽ����Au3+Ũ�Ƚ��ͣ�ƽ�����ƣ���������ˮ����3�֣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 -﮻�ʯ����Ҫ�ɷ�ΪLi2O
-﮻�ʯ����Ҫ�ɷ�ΪLi2O Al2O3
Al2O3 4SiO2��Ϊԭ������
4SiO2��Ϊԭ������ ��Li2CO3�Ĺ����������£�
��Li2CO3�Ĺ����������£�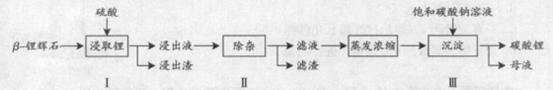
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ����¯�¶�/�� | 600 | 620 | 640 | 660 |
| ¯����CuSO4����������/% | 9.3 | 9.2 | 9.0 | 8.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
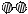 ��
�� ��
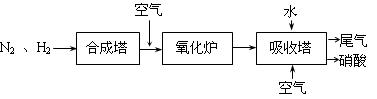
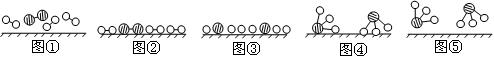
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� ��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� �� 2NH3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK��
2NH3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO3(��) | B��SO2 | C��H2S | D��NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
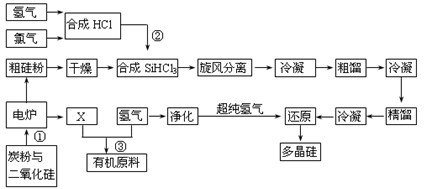
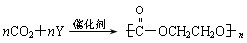 ����Y�Ľṹ��ʽΪ������������������������
����Y�Ľṹ��ʽΪ������������������������
 Si��s����4HCl(g)��
Si��s����4HCl(g)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
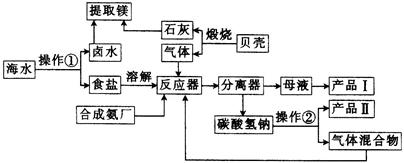
 ʽ)��
ʽ)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
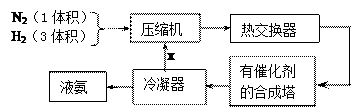
| A��N2��H2 | B������ | C��N2 | D��H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ⷨ | B�����ȷ�Ӧ�� |
| C��������CO��ԭ�� | D���ȷֽⷨ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com