
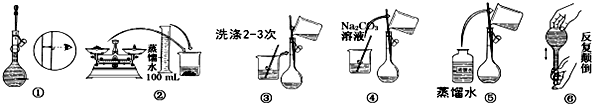
���� ��1����������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ����Ҫ��������
��2������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ݴ�����
��3���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��4������m=CVM������Ҫ����̼���Ƶ�����������Ũ��Һ���ƣ���������Һϡ�������������ʵ����ʵ������������ҪŨ��Һ�������
��� �⣺��1��ʵ������Ҫ����500mL 0.10mol/L Na2CO3��Һ��Ӧѡ��500mL����ƿ���������裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��õ���������������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ����Ի�ȱ�ٵIJ�������Ϊ��500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��2������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ�IJ�������Ϊ���ڢܢۢݢ٢ޣ�
�ʴ�Ϊ���ڢܢۢݢ٢ޣ�
��3���ٳ���ʱ���á��������������������ƽԭ�������̵�����=���̵�����+���������������ʵ�ʳ�ȡ������=���������-�������������ȡ�����ʵ�����ƫС�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��ת����Һ��û��ϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��������ƿ��ˮ����ʱ����Һ�棬������Һ���ƫС����Һ�����ʵ���Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��ҡ�Ⱥ�Һ���½����ټ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��4��ʵ������Ҫ����500mL 0.10mol/L Na2CO3��Һ��Ӧѡ��500mL����ƿ��ʵ������500mL��Һ����Ҫ̼���Ƶ�����m=0.10mol/L��106g/mol��0.5L=5.3g��
������Ũ��Һϡ�ͣ�����Ҫ��ȡ2mol/L Na2CO3��Һ���ΪV����������Һϡ�������������ʵ����ʵ��������V��2mol/L=0.10mol/L��500mL�����V=25.0mL��
�ʴ�Ϊ��5.3��25.0��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�����ע����������һ�����ʵ���Ũ�ȵ���Һ�ķ�������ȷ�������ķ����뼼�ɣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH�TCHCH��CH3��2 | B�� | ��CH3��2C�TCHCH2CH3 | ||
| C�� | CH3CH�TC��CH3��CH2CH3 | D�� | CH2�TCH CH2CH��CH3��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩ���ױ�����������е�����ԭ�Ӷ���ͬһƽ���� | |
| B�� | ��ȥ�����е���ϩʱ��ͨ���������Ӵ������� | |
| C�� | C3H8�Ķ��ȴ��ﹲ��3�� | |
| D�� | �Ҵ������ᡢ�����������ܷ���ȡ����Ӧ�����������е�����������ñ���Na2CO3��Һ��ȥ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH2O | B�� | CH3CHO | C�� | CH3CH2CHO | D�� | CH2�TCH-CHO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ͳ� | B�� | ţ�� | C�� | ���� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ȼ��� | B�� | �Ȼ��� | C�� | ���軯�� | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
| Ԫ�� | ���ֽṹ�ص� | �������� |
| X | X�ĵ�����˫ԭ�ӷ��ӹ��ɣ���������14 ������ | X�ж����������XO��XO2�� |
| Y | Yԭ�ӵĴ����������������������� ��һ�� | YԪ�����γɶ��ֵ��� |
| Z | Zԭ�ӵ���������������4 | ZԪ�ص���������ϼ���������ϼ۵Ĵ����͵���6 |
| W | ��������Ԫ�صļ������а뾶��С | W�ĵ��ʻ�ѧ������ϻ��ã���ֻ�賣�±��� |
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com