ij����С����ʵ��������ͼ��ʾ��װ�ý���ʵ�飬�ⶨ�������е�����ԭ�Ӹ����ȣ�
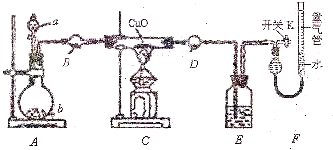
ʵ���У������Ƶõİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ����Ӧ��ɺ�ɫ������ͭת��Ϊ��ɫ��ͭ��
��ش��������⣺
��1��д������a�����ƣ�
��
��2��װ��B��D�е�ҩƷ��ͬ������Ϊ��
��
��3��д����һ��ʵ�����Ʊ������Ļ�ѧ��Ӧ����ʽ
��
��4��װ��C�з�����Ӧ�Ļ�ѧ��Ӧ����ʽΪ
���˷����а�����N
xH
y��ʾ����
��5����������ԭ���ס�����С�����ѡ�������ַ�����
| ʵ������1 | ʵ������2 | ʵ������3 |
| ��С�� | ��Ӧǰ����ͭ������Ϊm1g | ����ͭ��Ӧ��ʣ����������Ϊm2g | ���ɵ����ڱ�״���µ����ΪV1L |
| ��С�� | ϴ��ǰװ��D������Ϊm3g | ϴ����װ��D������Ϊm4g | ���ɵ����ڱ�״���µ����ΪV2L |
��С�����������ݼ�����������е�����ԭ�Ӹ���֮��Ϊ
��
��С�����������ݼ�����������е�����ԭ�Ӹ���֮��Ϊ
��







 ����ϩ���Ľṹʽ�����У�������
����ϩ���Ľṹʽ�����У�������