
(8��)���мס��ҡ�������ͬѧ�ֱ����Fe(OH)3������Ʊ�ʵ�顣
��ͬѧ����1 mol��L��1��FeCl3��Һ�м�����NaOH��Һ��
��ͬѧ��ֱ�Ӽ��ȱ���FeCl3��Һ��
��ͬѧ����25 mL��ˮ����μ���5��6��FeCl3������Һ�������������Һ�ʺ��ɫ��ֹͣ���ȡ�
�Իش��������⣺
�����в�����ȷ��ͬѧ��___ _____��
��֤����Fe(OH)3�����������õĽ���������______ __��
���ڽ����м���������Һ������෴��ɵĽ�������ʹ������������������ͬѧ�������Ƶõ�Fe(OH)3�������ʵ�飺
�� ����װ��U�ι��ڣ���ʯī���缫��ͨ��һ��ʱ��������Դ���������ĵ缫����������ɫ��������Fe(OH)3��������__________(���������)��ɡ�
�� �������м��뱥��Na2SO4��Һ�������������� ��
���������м���Ũ������Һ�������������� ��
(4)Fe(OH)3�����Ʊ��Ļ�ѧ����ʽΪ ��
��8�֣�ÿ��1�֣��ۺͷ���ʽ��2�֣�(1)�� ��(2)�������ЧӦ��
(3)���������к��ɫ�������� ���к��ɫ�������ɼ�������������ܽ⣨��Һ���ɫ��
(4) FeCl3+3H2O Fe(OH)3(����)
+3HCl
Fe(OH)3(����)
+3HCl
��������
�����������1���Ʊ�������������ķ��������ˮ����μ���FeCl3������Һ�������������Һ�ʺ��ɫ��ֹͣ���ȼ��ɣ����Ա�ͬѧ��ȷ��
��2�������ܲ��������ЧӦ���ݴ˿��Լ���
��3�������Դ���������ĵ缫����������ɫ���֪ʶ��������������Ľ����Ǵ�����ɵġ�
�ڽ���͵���ʻ�ϻᷢ���۳�������к��ɫ���������������ɡ�
������Ҳ�ǵ���ʣ������������ԣ����к����������������������к��ɫ�������ɼ�������������ܽ⡣
��4��)Fe(OH)3�����Ʊ��Ļ�ѧ����ʽΪFeCl3+3H2O Fe(OH)3(����) +3HCl��
Fe(OH)3(����) +3HCl��
���㣺������������������Ʊ������ʵ��й��жϺ�Ӧ��
����������������������ɢϵ�ı��������Ƿ�ɢ������ֱ����С��ͬ�����������ЧӦ���������ЧӦ������������Һ�ͽ��塣�����ѶȲ���


 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ����һ�и�һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(8��)���мס��ҡ�������ͬѧ�ֱ����Fe(OH)3������Ʊ�ʵ�顣
��ͬѧ����1 mol��L��1��FeCl3��Һ�м�����NaOH��Һ��
��ͬѧ��ֱ�Ӽ��ȱ���FeCl3��Һ��
��ͬѧ����25 mL��ˮ����μ���5��6��FeCl3������Һ�������������Һ�ʺ��ɫ��ֹͣ���ȡ�
�Իش��������⣺
�����в�����ȷ��ͬѧ��___ _____��
��֤����Fe(OH)3�����������õĽ���������______ __��
���ڽ����м���������Һ������෴��ɵĽ�������ʹ������������������ͬѧ�������Ƶõ�Fe(OH)3�������ʵ�飺
�� ����װ��U�ι��ڣ���ʯī���缫��ͨ��һ��ʱ��������Դ���������ĵ缫����������ɫ��������Fe(OH)3��������__________(���������)��ɡ�
�� �������м��뱥��Na2SO4��Һ�������������� ��
���������м���Ũ������Һ�������������� ��
(4)Fe(OH)3�����Ʊ��Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�ij���ij�ص���и�����ѧ��1�·�ģ���⻯ѧ�Ծ����������� ���ͣ������
(8��)���мס��ҡ�������ͬѧ�ֱ����Fe(OH)3������Ʊ�ʵ�顣
��ͬѧ����1 mol��L��1��FeCl3��Һ�м�����NaOH��Һ��
��ͬѧ��ֱ�Ӽ��ȱ���FeCl3��Һ��
��ͬѧ����25 mL��ˮ����μ���5��6��FeCl3������Һ�������������Һ�ʺ��ɫ��ֹͣ���ȡ�
�Իش��������⣺
�����в�����ȷ��ͬѧ��___ _____��
��֤����Fe(OH)3�����������õĽ���������______ __��
���ڽ����м���������Һ������෴��ɵĽ�������ʹ������������������ͬѧ�������Ƶõ�Fe(OH)3�������ʵ�飺
�� ����װ��U�ι��ڣ���ʯī���缫��ͨ��һ��ʱ��������Դ���������ĵ缫����������ɫ��������Fe(OH)3��������__________(���������)��ɡ�
�� �������м��뱥��Na2SO4��Һ�������������� ��
���������м���Ũ������Һ�������������� ��
(4)Fe(OH)3�����Ʊ��Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�ij��и�����ѧ��1�·�ģ���⻯ѧ�Ծ��������棩 ���ͣ������
(8��)���мס��ҡ�������ͬѧ�ֱ����Fe(OH)3������Ʊ�ʵ�顣
��ͬѧ����1 mol��L��1��FeCl3��Һ�м�����NaOH��Һ��
��ͬѧ��ֱ�Ӽ��ȱ���FeCl3��Һ��
��ͬѧ����25 mL��ˮ����μ���5��6��FeCl3������Һ�������������Һ�ʺ��ɫ��ֹͣ���ȡ�
�Իش��������⣺
�����в�����ȷ��ͬѧ��___ _____��
��֤����Fe(OH)3�����������õĽ���������______ __��
���ڽ����м���������Һ������෴��ɵĽ�������ʹ������������������ͬѧ�������Ƶõ�Fe(OH)3�������ʵ�飺
�� ����װ��U�ι��ڣ���ʯī���缫��ͨ��һ��ʱ��������Դ���������ĵ缫����������ɫ��������Fe(OH)3��������__________(���������)��ɡ�
�� �������м��뱥��Na2SO4��Һ�������������� ��
���������м���Ũ������Һ�������������� ��
(4)Fe(OH)3�����Ʊ��Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����8�֣����мס��ҡ����������־���������ͼ��ʾ����
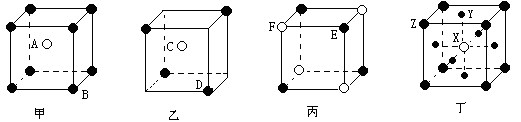
����֪��������A��B�����Ӹ�����Ϊ_______���Ҿ���Ļ�ѧʽΪ________��������Ļ�ѧʽΪ_________��������Ļ�ѧʽΪ___________������ѧʽ����ĸ����ĸ˳�����У�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com