
KI��Һ����������������������Ӧ�� ��������ʵ���¼��
| ʵ���� | �� | �� | �� | �� | �� |
| �¶ȣ��棩 | 30 | 40 | 50 | 60 | 70 |
| ��ɫʱ�䣨s�� | 160 | 80 | 40 | 20 | 10 |
�ش��������⣺
��1���÷�Ӧ�����ӷ���ʽΪ ��
��2����ʵ���Ŀ���� ��
��3��ʵ���Լ�����1 mol/L KI��Һ��0.1 mol/L H2SO4��Һ�⣬����Ҫ���Լ��� ��ʵ������Ϊ ��
��4������ʵ������г�����Ҫ��3���������⣬��������Ʋ������ (����ĸ)��
A���¶� B���Լ���Ũ�� C���Լ�������(���) D���Լ����ӵ�˳��
��5��������ʵ���¼�ɵó��Ľ����� ��
��6����Ҫ�������ԶԷ�Ӧ���ʵ�Ӱ���̽��ʵ�飬����ȡ�Ĵ�ʩ�� ��
��1��4H+ + 4I- + O2== 2I2+ 2H2O ��3�֣�
��2��̽���¶ȶԷ�Ӧ���ʵ�Ӱ�졣(̽���¶�����Һ��ɫʱ��Ĺ�ϵ)��2�֣�
��3��������Һ��2�֣��� ��ɫ��Һ����ɫ����2�֣�
��4��CD ��2�֣�
��5��ÿ����10�棬��Ӧ��������Լ2������2�֣����ش������������䣬�¶�Խ�ߣ���ѧ��Ӧ����Խ�����Һ��ɫʱ��Խ�̸�1�֣�
��6����������ʵ���������䣬���ò�ͬŨ�ȵ�������Һ���жԱ�ʵ�顣��2�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ������ʵ����ѧ������ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ������
(14��) ±�����±�����ڹ�ҵ������������Ҫ�����á�ijС��Ϊ̽������һЩ�ε����ʣ��������ϲ�����ʵ�顣�����������£�
�� BrO3�� + 6I�� + 6H+ = 3I2 + Br��+ 3H2O �� 2BrO3�� + I2 = 2IO3�� + Br2
�� IO3�� + 5I�� + 6H+ = 3I2 + 3H2O �� 2IO3�� + 10Br��+ 12H+ = I2 + 5Br2 + 6H2O
ʵ�����£�
| ���� | ���� |
| ��.��ʢ��30 mL 0.2 mol��L-1 KI��Һ����ƿ�����ε��뼸�ε�����Һ������ϡ���ᣬ���õζ�����μ���KBrO3��Һ | ����KBrO3��Һ���룬��Һ����ɫ��Ϊ��ɫ������,���ձ��ֲ��� |
| ��.������������Һ�е���KBrO3��Һ | ��Һ����ɫ����ȥ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ�߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ������
KI��Һ����������������������Ӧ�� ��������ʵ���¼��
|
ʵ���� |
�� |
�� |
�� |
�� |
�� |
|
�¶ȣ��棩 |
30 |
40 |
50 |
60 |
70 |
|
��ɫʱ�䣨s�� |
160 |
80 |
40 |
20 |
10 |
�ش��������⣺
��1���÷�Ӧ�����ӷ���ʽΪ ��
��2����ʵ���Ŀ���� ��
��3��ʵ���Լ�����1 mol/L KI��Һ��0.1 mol/L H2SO4��Һ�⣬����Ҫ���Լ��� ��ʵ������Ϊ ��
��4������ʵ������г�����Ҫ��3���������⣬��������Ʋ������ (����ĸ)��
A���¶� B���Լ���Ũ�� C���Լ�������(���) D���Լ����ӵ�˳��
��5��������ʵ���¼�ɵó��Ľ����� ��
��6����Ҫ�������ԶԷ�Ӧ���ʵ�Ӱ���̽��ʵ�飬����ȡ�Ĵ�ʩ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ������ѧ�ڶ������Ի�ѧ�Ծ��������棩 ���ͣ������
��.±�����±�����ڹ�ҵ������������Ҫ�����á�ijС��Ϊ̽������һЩ�ε���
�ʣ��������ϲ�����ʵ�顣�����������£�
�� BrO3�� + 6I�� + 6H+ = 3I2 + Br��+ 3H2O �� 2BrO3�� + I2 = 2IO3�� + Br2
�� IO3�� + 5I�� + 6H+ = 3I2 + 3H2O �� 2IO3�� + 10Br��+ 12H+ = I2 + 5Br2 + 6H2O
ʵ�����£�
|
���� |
���� |
|
��.��ʢ��30 mL 0.2 mol��L-1 KI��Һ����ƿ�����ε��뼸�ε�����Һ������ϡ���ᣬ���õζ�����μ���KBrO3��Һ |
����KBrO3��Һ���룬��Һ����ɫ��Ϊ��ɫ������,���ձ��ֲ��� |
|
��.������������Һ�е���KBrO3��Һ |
��Һ����ɫ����ȥ |
��ش�
��1�����������еķ�Ӧ��~�ܲ������ѧ֪ʶ���ж�IO3����BrO3����I2��Br2����������ǿ������˳���� ��KBrO3��Һ��KBr��Һ�����������·�Ӧ�����ӷ���ʽ�� ��
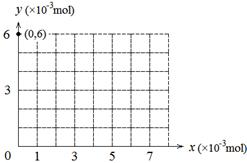
��2������y��ʾ��ƿ�к������ʵ����ʵ��� ����x��ʾ������KBrO3�����ʵ���������ͼ�л�����������ʵ�������y��x�ı仯���ߣ�Ҫ����ͼ�б���յ����꣩��

��.��̼�����к��������������ƣ��ס�����λͬѧ����ȡһ�������ĸ���Ʒ����������ͼ��ʾ�����ⶨ��Ʒ�Ĵ��ȡ�����������˳��
��ͬѧ���ݡ��ࡪ�ۡ��ߡ��ܣ� ��ͬѧ���ݡ��ۡ��ڡ�

��֪����̼���ƣ�Na2CO4�����������Ʒֱ������ϡ���ᷴӦ�Ļ�ѧ����ʽ���£�
2Na2CO4��2H2SO4=2Na2SO4��2CO2����O2����2H2O;
2Na2O2��2H2SO4=2Na2SO4��O2����2H2O��
��1����ͬѧ��ͨ��ʵ���õ�������____________����ѡ�õ�װ��________������ţ���û�б�Ҫ�ġ�
��2����ͬѧ��ͨ��ʵ���õ�������________________��������Ϊ������õ����ݼ������ʵ��������ƫ�ߣ�ԭ����________________�� Ϊ�˲��ȷ��ʵ�����ݣ����㽫��ͬѧ��ʵ����ƽ��иĽ���д������ѡ������������˳��ÿ���������ʹ��һ�Σ�Ҳ���Բ��ã���________________������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ѡ����
�ⶨ��I��Ũ�Ⱥ�С�ĵ⻯����Һʱ�����ñ�����Ӧ���л�ѧ�Ŵ������ԭ��Һ�еĵ����ӵ�Ũ�ȡ���Ҫ�������£�����Ŵ�ǰ����Һ�����ȣ�
���ڽ�������Һ�У����彫������I��������IO3���������������ȥ��
���ټ��������KI�������������£�ʹIO3����ȫת����I2��
�۽��������ɵĵ���ȫ��ȡ�����½��仹ԭΪI�������ӷ���ʽΪ��
N2H4��2I2 === 4 I����N2��4H��
�ܽ����ɵ�I������ȡ��ˮ����âٷ�������
�ݽ��ܵõ�����Һ�м���������KI��Һ�����������ữ��
���ݷ�Ӧ�����Һ�Ե�����ָʾ������Na2S2O3����Һ�ζ�����ѧ����ʽΪ��
2 Na2S2O3 ��I2 === Na2S4O6��2NaI
���������Ŵ����Һ��I��Ũ��Ϊԭ��Һ��I��Ũ�ȵģ�����������
A��6�������������� B��8���������������� C��18������������������ D��36��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com