
��ѧ������Ӧ�Ļ�ѧ����ʽ��A��B��X��Y��H2O��δ��ƽ����Ӧ������ȥ��������A��B�����ʵ���֮��Ϊ1:4����ش�
��1����Y�ǻ���ɫ���壬��Y�ĵ���ʽ�� _______ ���÷�Ӧ�Ļ�ѧ����ʽ��_______��
��2����AΪ�ǽ������ʣ���������ԭ�Ӻ��������������Ǵ�����������2����B����ҺΪijŨ�ᣬ��Ӧ���������뻹ԭ�������ʵ���֮����____________________��
��3����AΪ�������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ�С�
��AԪ�������ڱ��е�λ����_______�����������ں��壩��Y�Ļ�ѧʽ��_______��
�ں�a mol X����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X��_______ mol��
��4����A��B��X��Y��Ϊ�������A��Һ�м��������ữ��AgNO3��Һ��������ɫ������B����ɫΪ��ɫ����A��B�����ʵ���֮��1:4ǡ�÷�Ӧ�÷�Ӧ�Ļ�ѧ����ʽ��_______��
 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
| ||
| ||
| Cu |
| Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
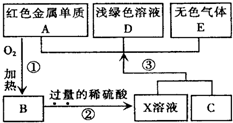 A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
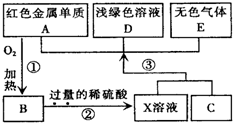
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡģ���� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ�����ʡ�Ƹ���ޭ������ʱ����ѧ��һ���£���ĩ��ѧģ���Ծ��������棩 ���ͣ������
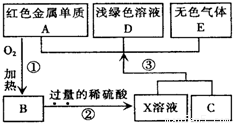
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com