
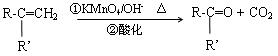 ��R��R����ʾ����������ţ�
��R��R����ʾ����������ţ�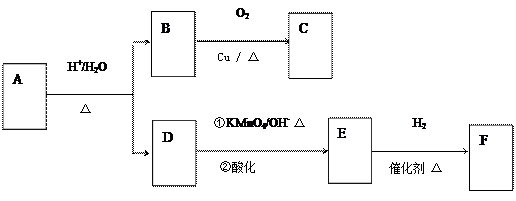
 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 rdR.Schrock��ͬ��á��Ա�����
rdR.Schrock��ͬ��á��Ա����� ����ϩ�����ֽⷴӦ�����о���Ӧ���������Ĺ��ס���֪ϩ���Ľ��渴�ֽⷴӦ����Ϊ˫�����ѣ���λ���ӡ��ɱ�ʾΪ��
����ϩ�����ֽⷴӦ�����о���Ӧ���������Ĺ��ס���֪ϩ���Ľ��渴�ֽⷴӦ����Ϊ˫�����ѣ���λ���ӡ��ɱ�ʾΪ��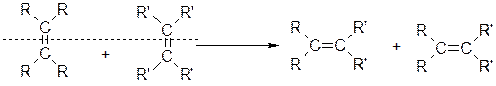
 R-CHCl-CH=CH2+HCl��
R-CHCl-CH=CH2+HCl�� �ȣ�FҲ������֬ˮ��õ����л���R�ĺϳ�·�����£�
�ȣ�FҲ������֬ˮ��õ����л���R�ĺϳ�·�����£�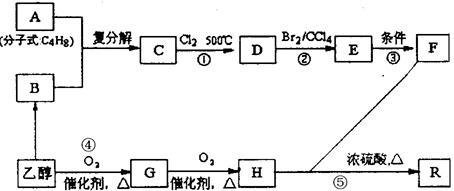
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
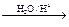 R��CH2CHO + R��OH������ϩ����A��ʽ��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4 ���л���B��ʽ��Ϊ60����صķ�Ӧ���¡���ش��������⣺
R��CH2CHO + R��OH������ϩ����A��ʽ��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4 ���л���B��ʽ��Ϊ60����صķ�Ӧ���¡���ش��������⣺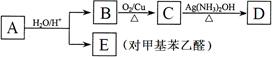
 _______________________��
_______________________�� ___________________��
___________________��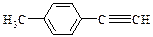 ����һ��·�����£�
����һ��·�����£�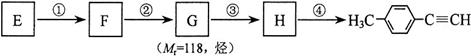
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
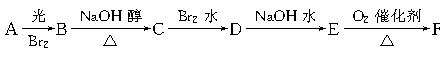
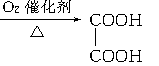
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �ṹ | ������ֻ����һ��������������ֻ��һ��ȡ��������֧������ȡ�����Ϻ���̼̼˫�� |
| ���� |  ����ʹ��ˮ������ѧ��Ӧ����ɫ ����ʹ��ˮ������ѧ��Ӧ����ɫ����NaOH��Һ������ˮ�ⷴӦ���ɾ��о綾�Ĵ������� |
 ģ������ͼ��ʾ��ͼ��������֮������߱�ʾ������˫������
ģ������ͼ��ʾ��ͼ��������֮������߱�ʾ������˫������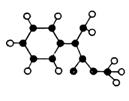

 E��F�ķ�Ӧ������ ��
E��F�ķ�Ӧ������ ��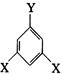 ��ʾ������X��Y����ΪH������д����������ͨʽ���ܷ���������Ӧ�������������ʵĽṹ��ʽ��
��ʾ������X��Y����ΪH������д����������ͨʽ���ܷ���������Ӧ�������������ʵĽṹ��ʽ�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
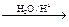 R��CH2CHO + R��OH
R��CH2CHO + R��OH 
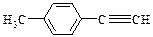 ����һ��·�����£�
����һ��·�����£�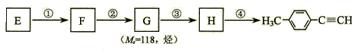
| ��� | �����Լ�����Ӧ���� | ��Ӧ���� |
| �� | | |
| �� | | |
| �� | | |
| �� | | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

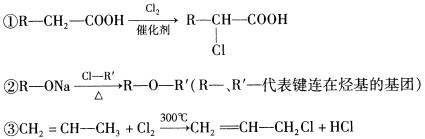
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
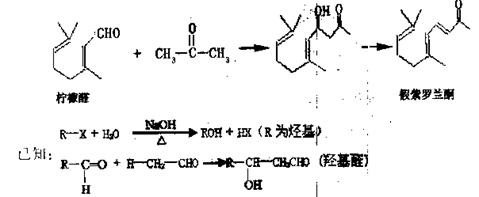
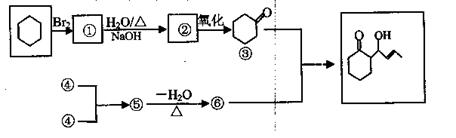
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
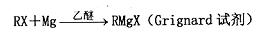
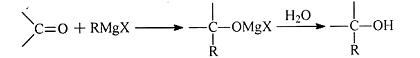
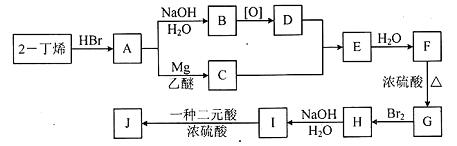
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com