
���� ��1�������谷�����У������ϵ�Nԭ�ӳ�3���� ������һ���µ��Ӷԣ����ϵ�Nԭ�ӳ�2���� ������һ���µ��Ӷԣ��ӻ������=�� ����+�µ��Ӷ������ݴ�ȷ��Nԭ���ӻ���ʽ�����������谷�����к��Ц� �����㣻
��2��Ԫ��λ�ڵ�������VIII�壬���̬ԭ�ӵ�δ�ɶԵ��������̬̼ԭ�ӵ�δ�ɶԵ�������ͬ��Cԭ�ӵĵ����Ų�Ϊ1s22s22p2��δ�ɶԵ�����Ϊ2�����Ԫ��ΪNi��
��3��Ni�ļ۵�����Ϊ10��ÿ�������ṩһ�����Ӷԣ�����10+2n=18���㣻
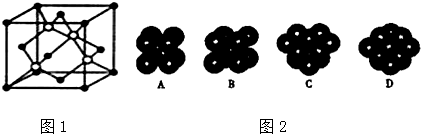
��4����ͼ2��֪��̼ԭ��Ϊ���������ѻ���ΪABC�Ͷѻ���
��5���Զ���Cԭ���о�����֮�����Cԭ��λ�������ϣ�ÿ������ԭ��Ϊ12���湲�ã����ݾ�̯�����㾧����C��Siԭ����Ŀ���������㾧���к���C��Siԭ������������㾧��������������Ŀռ�������=$\frac{������C��Siԭ�������}{�������}$��100%��
��� �⣺��1�������谷�����У������ϵ�Nԭ�Ӻ���3�� �� ����һ���µ��Ӷԣ����Բ�ȡsp3�ӻ������ϵ�Nԭ�Ӻ���2�� �� ����һ���µ��Ӷԣ����Բ�ȡsp2�ӻ���һ�������谷�����к���15���� ��������1mol�����谷������ �� ��Ϊ15mol��
�ʴ�Ϊ��sp2��sp3��15��
��2��Ԫ��λ�ڵ�������VIII�壬���̬ԭ�ӵ�δ�ɶԵ��������̬̼ԭ�ӵ�δ�ɶԵ�������ͬ��Cԭ�ӵĵ����Ų�Ϊ1s22s22p2��δ�ɶԵ�����Ϊ2�����Ԫ��ΪNi�����̬ԭ�ӵ�M������Ų�ʽΪ3s23p63d8��
�ʴ�Ϊ��3s23p63d8��
��3�����ݹ���ԭ��֪Ni�Ļ�̬��������Ų�Ϊ��1s22s22p63s23p63d84s2��Ni�ļ۵�����Ϊ10��ÿ�������ṩһ�����Ӷԣ���10+2n=18����n=4��
�ʴ�Ϊ��4��
��4����ͼ2��֪��̼ԭ��Ϊ���������ѻ���ΪABC�Ͷѻ���ѡ��D���ϣ�
�ʴ�Ϊ��D��
��5���Զ���Cԭ���о�����֮�����Cԭ��λ�������ϣ�ÿ������ԭ��Ϊ12���湲�ã�����̼ԭ�Ӿ����������ȵ�̼ԭ����12����
�㾧����Cԭ����Ŀ=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Siԭ����Ŀ=4�������߳�Ϊa cm�������Ϊa3cm3��̼ԭ��ֱ��Ϊb cm������Cԭ�������=4��$\frac{4}{3}$���С���$\frac{b}{2}$��3cm3=$\frac{2}{3}$��b3cm3����ԭ��ֱ��Ϊc cm������Siԭ�������=4��$\frac{4}{3}$���С���$\frac{c}{2}$��3cm3=$\frac{2}{3}$��c3cm3���ʾ�����C��Siԭ�������=$\frac{2}{3}$��b3cm3+$\frac{2}{3}$��b3cm3=$\frac{2}{3}$�У�b3+c3��cm3���ʾ����Ŀռ�������=$\frac{\frac{2}{3}�У�{b}^{3}+{c}^{3}��c{m}^{3}}{{a}^{3}c{m}^{3}}$��100%=$\frac{2�У�{b}^{3}+{c}^{3}��}{3{a}^{3}}$��100%��
�ʴ�Ϊ��$\frac{2�У�{b}^{3}+{c}^{3}��}{3{a}^{3}}$��100%��
���� ���⿼�龧���ṹ����㡢�ӻ����ۡ���������Ų�����ѧ���ȣ���Ҫѧ���������õĿռ��������������������4����5��Ϊ�״��㡢�ѵ㣬��Ŀ�Ѷ��еȣ�


 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ca��HCO3��2��Һ�м���������NaOH��Һ��Ca2++HCO3-+OH-��CaCO3��+H2O | |
| B�� | ��NH4Al��SO4��2��Һ�е���Ba��OH��2ʹSO42��ǡ����ȫ��Ӧ��2Ba2++4OH-+Al3++2SO42-��BaSO4��+AlO2-+2H2O | |
| C�� | ������CO2ͨ�뱥��̼������Һ�У�CO2+CO32-+H2O��2HCO3- | |
| D�� | ��Fe2��SO4��3��Һ�м������Na2S��Һ��2Fe3++3S2-��2FeS��+S�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2.8g | B�� | 5.6g | C�� | 8.4g | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ͭ���ء��������Ԫ�صĻ�����������������̫���ܵ�ص���Ҫ���ϣ���ش�
ͭ���ء��������Ԫ�صĻ�����������������̫���ܵ�ص���Ҫ���ϣ���ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����������ѡ���Ǵ���� | B�� | �ҵ�������ȷ | ||
| C�� | ������������ѡ������ȷ�� | D�� | ����������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������������ѧ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ���������£�CO��CH4ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ
2CO(g)+O2(g)=2CO2(g)��H=-566kJ/mol
CH4(g)+2O2(g)=CO2(g)+2H2O(l)��H=-890kJ/m ol
ol
��1molCO��3molCH4��ɵĻ������������������ȫȼ��ʱ���ͷŵ�����Ϊ
A��2953kJ B��2912kJ C��3236kJ D��3867kJ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com