
����Ŀ����0.100molL-1������ζ�10ml0.100molL-1��Na2CO3��Һ����ҺpHֵ��������������Ĺ�ϵ��ͼ��ʾ����֪����ʱ����CO2��pHΪ3.9��0.05molL-1NaHCO3��pHԼΪ8.3������˵������ȷ���ǡ�
��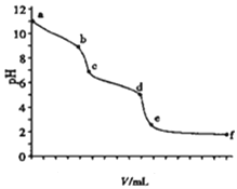
A. �Է�̪Ϊָʾ������Һ��ɫ����ͻ��ʱ��b�㣩��Na2CO3ת��ΪNaHCO3
B. �Լ���Ϊָʾ������Һ��ɫ����ͻ��ʱ������������20.00ml
C. ��ҺpH=7��c�㣩��̼������ȫת��ΪNaCl
D. ce�ζ�Ӧ��Һ��CO2��NaHCO3����
���𰸡�C
����������̪�ı�ɫ��Χ��8-10����Һ��ɫ����ͻ��ʱNa2CO3ת��ΪNaHCO3����A��ȷ�����ȵı�ɫ��Χ��3.1-4.4�����Լ��ȱ�ɫ��Һ�����ԣ�̼��������ȫת��Ϊ�Ȼ��ƣ�10ml 0.100mol/L��Na2CO3��Һǡ����ȫ��Ӧ��������20.00ml����B��ȷ����pH=7ʱ����Һ��ʾ���ԣ������Ӻ�����������Ũ����ȣ���ʱ��Һ�л�����̼������Ӻ�̼��������ӣ����������Ӵ���������Ũ�ȣ���C����; ����pH�仯���ߣ����Կ���c��d�Σ���������ļ��룬pH���ٱ仯�����Ըý�����ʣ�̼࣬���ƺ�̼��������ȫ���ĵ��ˣ�����ce�ζ�Ӧ��Һ��CO2��NaHCO3���棬��D��ȷ��


 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��T��ʱ��Ũ�Ⱦ�Ϊ1mol/L����������HA��HB�в��ϼ�ˮϡ�ͣ�����pH�������ⶨ��ҺpH��������ҺpH������(2pH)����ҺŨ�ȵĶ���(lgc)�Ĺ�ϵ��ͼ��ʾ�����������������
��֪����HA�ĵ���ƽ�ⳣ����![]()
��pKa=-lgKa
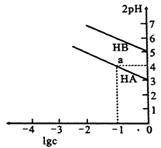
A. ���ԣ�HA>HB
B. a���Ӧ����Һ�У�c(HA)=0.1mol/L��c(H+)=0.01mol/L
C. T��ʱ������HB��pKa��5
D. �����Ka����ҺŨ�ȵĽ��Ͷ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ȱ���ȼ�ϡ�ҽҩ���л��ϳɵ��м��壬����Ҫ���л�������Ʒ��ʵ������ȡ�ȱ���װ����ͼ��ʾ(���Ⱥ̶�������װ����ȥ)���ش��������⣺
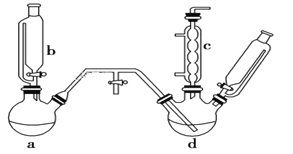
(1)a��b������ϳ���ȡ������װ�ã���Ӧ������ȣ���aװ���еĹ��巴Ӧ�������________(����ĸ���)��
A��MnO2 B��KMnO4 C��K2Cr2O7
(2)������ͨ�뷴Ӧ��d��(dװ����FeCl3�ͱ�)������ά�ַ�Ӧ�¶�40��60 �棬�¶ȹ������ɹ���Ķ��ȱ�����d���ȵķ�����______________________��
(3)װ��c���ڵ�����ɷ���HCl��Cl2��ˮ������_____��
(4)��ȡ�ȱ��Ļ�ѧ����ʽΪ_______________________ ��
(5)װ��d�еķ�Ӧ��ɺ�ҵ��Ҫ����ˮϴ����ϴ��ʳ�θ����������
�ټ�ϴ֮ǰҪˮϴ����Ŀ����______________________________��
����10%NaOH��Һ��ϴʱ����������ԭ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ_____________________��
(6)���ɵ������д���HCl��H2O���������壬����Ҫ����һ��װ�ó�ȥˮ�������뻭����װ��ͼ�������������Լ�________��
(7)��ҵ�����б�����ʧ������±���ʾ��
��Ŀ | ���ȱ� | �Ȼ�β�� | ���� | ��Ʒ | ��ȷ������ | �ϼ� |
����ʧ��kg/t | 11.7 | 5.4 | 20.8 | 2.0 | 49.3 | 89.2 |
��10t�����Ƶó�Ʒ�ȱ�________t(�г�����ʽ���ɡ��ȱ��ͱ�����Է��������ֱ���112.5��78)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ڹ�ũҵ�����ж�����ҪӦ�á�
(1)������(N2H4)����������ĵ��⻯�
��֪��4NH3(g)��3O2(g)![]() 2N2(g)��6H2O(g)����H1����541��8 kJ��mol��1����ѧƽ�ⳣ��ΪK1��N2H4(g)��O2(g)
2N2(g)��6H2O(g)����H1����541��8 kJ��mol��1����ѧƽ�ⳣ��ΪK1��N2H4(g)��O2(g)![]() N2(g)��2H2O(g)����H2����534 kJ��mol��1����ѧƽ�ⳣ��ΪK2������NH3��O2��ȡN2H4���Ȼ�ѧ����ʽΪ__________________���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��________(��K1��K2��ʾ)��
N2(g)��2H2O(g)����H2����534 kJ��mol��1����ѧƽ�ⳣ��ΪK2������NH3��O2��ȡN2H4���Ȼ�ѧ����ʽΪ__________________���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��________(��K1��K2��ʾ)��
(2)����2NO(g)��2CO(g)![]() N2(g)��2CO2(g)����һ���¶��£���1 L�ĺ����ܱ������г���0��1 mol NO��0��3 mol CO����Ӧ��ʼ���С�
N2(g)��2CO2(g)����һ���¶��£���1 L�ĺ����ܱ������г���0��1 mol NO��0��3 mol CO����Ӧ��ʼ���С�
��������˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����______(����ĸ����)��
A��c(CO)��c(CO2)
B�������л��������ܶȲ���
C��v(N2)����2v(NO)��
D�������л�������ƽ��Ħ����������
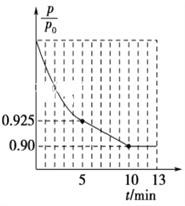
��ͼ1Ϊ�����ڵ�ѹǿ(p)����ʼѹǿ(p0)�ı�ֵ![]() ��ʱ��(t)�ı仯���ߡ�0��5min�ڣ��÷�Ӧ��ƽ����Ӧ����v(N2)��________��ƽ��ʱNO��ת����Ϊ________��
��ʱ��(t)�ı仯���ߡ�0��5min�ڣ��÷�Ӧ��ƽ����Ӧ����v(N2)��________��ƽ��ʱNO��ת����Ϊ________��
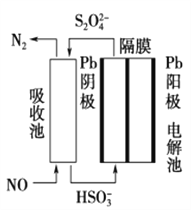
(3)ʹ�ü�ӵ绯ѧ���ɴ���ȼ�������е�NO��װ����ͼ��ʾ����֪���ص�����������Һ��pH��4��7֮�䣬д�������ĵ缫��Ӧʽ��____________________�������ӷ���ʽ��ʾ���ճ��г�ȥNO��ԭ��____________________________________________��
���𰸡� 4NH3(g)��O2(g)2N2H4(g)��2H2O(g)��H����526.2 kJ��mol��1 K1/K22 D 0��006 mol��L��1��min��1 80% 2HSO3-��2e����2H��===S2O42-��2H2O 2NO��2S2O42-��2H2O===N2��4HSO3-
��������(1)��4NH3(g)��3O2(g) ![]() 2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1����N2H4(g)��O2(g)
2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1����N2H4(g)��O2(g) ![]() N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2�����ݸ�˹���ɣ�����-����2�ã�4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=(��541.8kJ/mol)-(��534kJ/mol)��2=��526.2kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=
N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2�����ݸ�˹���ɣ�����-����2�ã�4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=(��541.8kJ/mol)-(��534kJ/mol)��2=��526.2kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=![]() ���ʴ�Ϊ��4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol��
���ʴ�Ϊ��4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol�� ![]() ��
��
(2)����2NO(g)��2CO(g) ![]() N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
��A��c(CO)=c(CO2)������ʾŨ�ȱ仯�������ж��Ƿ�Ϊƽ��״̬����A����B����Ӧ��������������䣬������䣬�����л��������ܶ�ʼ�ղ��䣬�����ж��Ƿ�Ϊƽ��״̬����B����C��v(N2)��=2v(NO)����ʾ��Ӧ����2v(N2)��=v(NO)�����ű�ʾ���淴Ӧ������ȣ���C����D���÷�Ӧ������������ʵ��������仯�ķ�Ӧ�������л�������ƽ��Ħ����������ʱ��ʾ��������ʵ������䣬 ˵����ƽ��״̬����D��ȷ����ѡD��
�����������ڵ�ѹǿ(P)����ʼѹǿ(P0)�ı�ֵ(P/P0)��ʱ��(t)�ı仯���ߣ�0��5min�ڣ�![]() =0.925�����ݰ���٤�����ɼ������ۣ�
=0.925�����ݰ���٤�����ɼ������ۣ�![]() =0.925��ƽ��ʱ
=0.925��ƽ��ʱ![]() =0.90��
=0.90��
2NO(g)�� 2CO(g) ![]() N2(g)��2CO2(g)
N2(g)��2CO2(g)
��ʼ(mol) 0.1 0.3 0 0
��Ӧ 2x 2x x 2x
5min��ƽ�� 0.1-2x 0.3-2x x 2x
5minʱ��![]() =0.925�����x=0.03mol��v(N2)=
=0.925�����x=0.03mol��v(N2)=![]() = 0.006mol��L��1��min��1��ƽ��ʱ��
= 0.006mol��L��1��min��1��ƽ��ʱ��![]() =0.90�����x=0.04mol��NO��ת����=
=0.90�����x=0.04mol��NO��ת����=![]() ��100%=80%���ʴ�Ϊ��0.006 mol��L��1��min��1��80%��
��100%=80%���ʴ�Ϊ��0.006 mol��L��1��min��1��80%��
(3)����������ԭ��Ӧ����������������ӣ��õ��ӣ����������������ӣ��缫��ӦʽΪ��2HSO3-+2e-+2H+�TS2O42-+2H2O����������������һ����������������ԭ��Ӧ�����ɵ��������ӷ�Ӧ����ʽΪ��2NO+2S2O42-+2H2O�TN2+4HSO3-���ʴ�Ϊ��2HSO3-+2H++2e-=S2O42-+2H2O��2NO+2S2O42-+2H2O=N2+4HSO3-��
�����͡������
��������
10
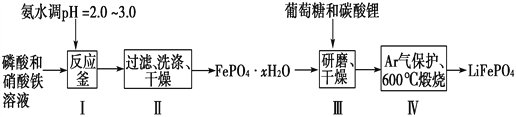
����Ŀ������ӵ����Ŀǰ������߱������Ķ��ε�ء�LiFePO4�ɼ���ظ��Ƶ����ϵ�İ�ȫ���ܣ��Ҿ�����Դ�ḻ��ѭ���������������Ѻõ��ص㣬������ӵ���������ϵ�����ѡ������LiFePO4��һ�ֹ���������ͼ��
��֪��Ksp(FePO4��xH2O)��1.0��10��15��Ksp[Fe(OH)3]��4.0��10��38��
(1)�ںϳ�������ʱ���������pH�Ŀ����ǹؼ������pH<1.9��Fe3����������ȫ��Ӱ����������pH��3.0������ܴ��ڵ�������________________��
(2)������У�ϴ����Ϊ�˳�ȥFePO4��xH2O���渽�ŵ�________�����ӡ�
(3)ȡ3��FePO4��xH2O��Ʒ���������³�����ղ���ᾧˮ������ʵ���������±���
ʵ����� | 1 | 2 | 3 |
����ʧ���������� | 19.9% | 20.1% | 20.0% |
����ʧ������������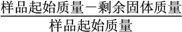 ��100%����x��_______(��ȷ��0.1)��
��100%����x��_______(��ȷ��0.1)��
(4)���������ĥ��������__________________________________��
(5)�ڲ������������LiFePO4��CO2��H2O�����������뻹ԭ�������ʵ���֮��Ϊ________��
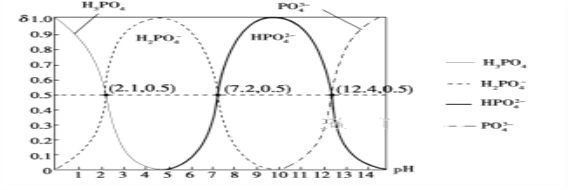
(6)H3PO4����Ԫ�ᣬ��ͼ�dz�������Һ�к����������ʵ�������(��)��pH�仯ʾ��ͼ����PO![]() ��һ��ˮ���ˮ�ⳣ��K1�ı���ʽΪ______��K1����ֵ��ӽ�______(����ĸ)��
��һ��ˮ���ˮ�ⳣ��K1�ı���ʽΪ______��K1����ֵ��ӽ�______(����ĸ)��
A��10��12.4����B��10��1.6 C��10��7.2 D��10��4.2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(G)���з��ɻ�����ζ,�����ڵ����ζʳƷ�㾫�����û�ױƷ�㾫��һ���÷�����A�ϳ�F�Ĺ���·������:

�ش���������:
��1��E�ṹ��ʽΪ____________��C��������____________
��2���ٵġ���Ӧ������Ϊ________;�ڵķ�Ӧ����Ϊ__________.
��3��F�����������ŵ�����Ϊ___________
��4��д��F![]() G��Ӧ�Ļ�ѧ����ʽ___________
G��Ӧ�Ļ�ѧ����ʽ___________
��5��F��ͬ���칹����,ͬʱ����������������__��(�����������칹);������FeCl3��Һ������ɫ���ڱ�����������ȡ��������һ��̼̼˫�������к˴Ź���������5���,�ҷ����֮��Ϊ1:2:2:2:3�Ľṹ��ʽΪ__________.
��6���۲챾��ϳ�·�ߵ��Լ�������,�������Ϻϳ�·���е������Ϣ����д����HCHO��CH3CHO���Ʊ�CH2=CHCH2OOCCH3�ĺϳ�·��ͼ��(��Ӧ�P����д�ɽṹ��ʽ)________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���밴Ҫ��ش���������:
I.25��ʱ�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L�ļ�������Һ��pH���:
��� | �� | �� | �� | �� |
��Һ | NH4Cl | CH3COONH4 | NaHCO3 | Na2CO3 |
pH | 5 | 7 | 8.4 | 11.6 |
��1�������ӷ���ʽ������Һ����ȥ�����۵�ԭ��________________________��
��2��д����Һ���е���غ�Ĺ�ϵʽ:_________________________________________��
��3��25��ʱ����Һ���У���ˮ���������c(OH-)=_______mol/L��
������Һ����Ka(CH3COOH )_____Kb(NH3��H2O) (����>������<������=��)��
II.�����������˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ���(��������Fe2O3��TiO2) ������ˮ�������з�Ӧ��
��1����֪: N2(g) + 3H2(g)![]() 2NH3(g) ��H=-92.4kJ��mol-1
2NH3(g) ��H=-92.4kJ��mol-1
2H2(g) + O2(g)= 2H2O (1) ��H =-571.6kJ��mol-1
��2N2(g) + 6H2O(1)![]() 4NH3(g) + 3O2(g) ��H=______��
4NH3(g) + 3O2(g) ��H=______��
��2��������(Si3N4)��һ�������մɲ��ϣ�������ʯӢ�뽹̿�ڸ��µĵ������У�ͨ�����·�Ӧ�Ƶ�: 3SiO2(s)+ 6C(s) + 2N2(g) ![]() Si3N4(s)+ 6CO(g)���÷�Ӧ��ƽ�ⳣ������ʽΪK=_________��
Si3N4(s)+ 6CO(g)���÷�Ӧ��ƽ�ⳣ������ʽΪK=_________��
��3����N2��H2Ϊ��Ӧ��(����ͼ)�������ữ��NH4Cl��ҺΪ����ʵ�ԭ��أ�a�缫�ĵ缫��ӦʽΪ:____________________________________________��
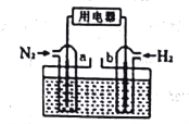
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
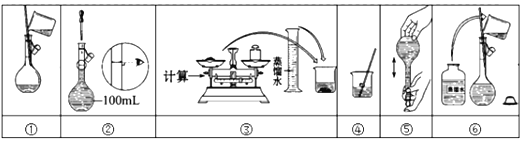
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش��������⣺

(1)�á�84����Һ�������ʵ���Ũ��Լ______mol��L-1(С�������һλ)��
(2)ijͬѧȡ100mL�á�84����Һ����ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na+)=______mol��L-1��
(3)��ͬѧ���ĸá�84����Һ�����䷽������NaClO��������80mL��NaClO��������Ϊ25%������Һ����Ҫ����NaClO���������Ϊ_______g������Ϊ�����˵IJ���ʾ��ͼ������ȷ����Ϊ_______________________��
(4)���ƹ��������и������������Һ���ʵ���Ũ���к�Ӱ�죨�ƫС������ƫ����Ӱ�족��
A����������ƽ���� NaClO����ʱ����������__________________��
B������ƿ������ˮϴ�Ӻ�δ���������������ˮ____________________��
C������ʱ����������ƿ�̶��߽��ж��� ___________________��
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����Ҵ���˵����ȷ����
A.Ħ������Ϊ46gB.����ɫ��ζ��Һ��
C.����������Ʒ�ӦD.75% (�������)���Ҵ���Һ������ҽ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��A��B��C��D�����ǵ�ԭ��������������A��Dͬ����������������Ϊ������AΪԪ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Bԭ�ӵ������������Ǵ�����������2����CΪ�ؿ��к�������Ԫ�ء���ش��������⣺
��1��CԪ�ص����ӽṹʾ��ͼ________��CԪ���γɵij���Һ̬������Ľṹʽ________��
��2��DԪ����Ԫ�����ڱ��е�λ��__________��д��D��C������ȼ���γɵĻ�����ĵ���ʽ__________��
��3��B��C�ֱ��γɵļ���̬�⻯���н��ȶ�����______������B�γɵļ���̬�⻯��Ŀռ�ṹ _______������B��C��Ӧ���ɵ�XY2�ͻ�����ĵ���ʽ______________��
��4������B�������������D������������Ӧ��ˮ�������Һ��Ӧ�����ӷ���ʽ__________________________________________________________________��
��5������ʽΪB6A14�����ʺ��ж���ͬ���칹�壬����һ��ͬ���칹���һ�ȴ���ֻ�����֣�д���������ʵĽṹ��ʽ______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com