
| A£®øł¾ŻĶ¼ĖłŹ¾Źż¾Ż¼ĘĖ揵ŃéÖŠŹ¹ÓƵÄNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ1mol/L |
| B£®øł¾ŻŠÅĻ¢·ÖĪöøĆČÜŅŗÖŠŃęÉ«·“Ó¦³ŹĻÖ×ĻÉ«µÄĄė×ÓµÄĪļÖŹµÄĮæÅضČĪŖ1mol/L |
| C£®ĮķČ”Ņ»¶ØĮæøĆČÜŅŗµĪ¼ÓŅ»¶ØĮæµÄBa(OH)2ČÜŅŗ£¬ÄÜŹ¹Al3+ŗĶSO42”„Ķ¬Ź±ĶźČ«³Įµķ |
| D£®NH4+”¢K+”¢Al3+”¢SO42”„ĖÄÖÖĄė×ÓµÄĪļÖŹµÄĮæÖ®±ČĪŖ£ŗ2£ŗ1£ŗ1£ŗ3 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®½«AlĢõĶ¶ČėNaOHČÜŅŗÖŠ£ŗAl£«OH££«H2O=AlO2”Ŗ£«H2”ü |
| B£®ĶČÜÓŚĻ”ĻõĖįÖŠ£ŗCu£«4H£«£«2NO3”Ŗ=Cu2£«£«2NO2”ü£«2H2O |
| C£®Ģ¼ĖįĒāøĘČÜŅŗÖŠ¼ÓČė¹żĮæµÄĒāŃõ»ÆÄĘČÜŅŗ£ŗHCO3”Ŗ£«OH£=CO32”Ŗ£«H2O |
| D£®ĻņĢ¼ĖįÄĘČÜŅŗÖŠÖšµĪ¼ÓČėÓėÖ®µČĢå»żµČĪļÖŹµÄĮæÅØ¶ČµÄĻ”“×Ėį£ŗCO32”Ŗ£«CH3COOH=CH3COO££«HCO3”Ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼ČÄÜČܽāAl£ØOH£©3£¬ÓÖÄÜČܽāH2SiO3µÄČÜŅŗ£ŗNa£«”¢Fe2£«”¢SO42-”¢Cl£ |
| B£®ÄÜŹ¹·ÓĢŖČÜŅŗ±äŗģµÄČÜŅŗ£ŗNa£«”¢NO3-”¢S2£”¢Br£ |
| C£®Ķ¶ČėĢśĘ¬ÄܲśÉśH2µÄĪŽÉ«ČÜŅŗ£ŗH£«”¢Mg2£«”¢SO42-”¢NO3- |
| D£®ŗ¬ÓŠ“óĮæFe3£«µÄČÜŅŗ£ŗNa£«”¢Al3£«”¢NO3-”¢SCN£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 ӢFe2+ӢFe3+ӢC
ӢFe2+ӢFe3+ӢC ӢS
ӢS ӢS
ӢS ӢCl-ӢN
”¢Cl-”¢N ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ,Č”øĆČÜŅŗ½ųŠŠŹµŃé,ŹµŃéÄŚČŻČēĻĀ:
ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ,Č”øĆČÜŅŗ½ųŠŠŹµŃé,ŹµŃéÄŚČŻČēĻĀ:
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

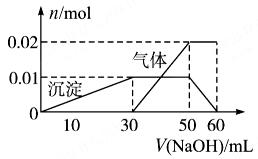
| A£®MµćŹ±Éś³ÉµÄCO2ĪŖ0mol |
| B£®Ō»ģŗĻČÜŅŗÖŠµÄCO32£ÓėAlO2£µÄĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ2 |
| C£®V1£ŗV2=1£ŗ4 |
| D£®aĒśĻß±ķŹ¾µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAlO2£+H+ + H2O£½Al(OH)3”ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®SO32- | B£®Na£« | C£®AlO2- | D£®SO42- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
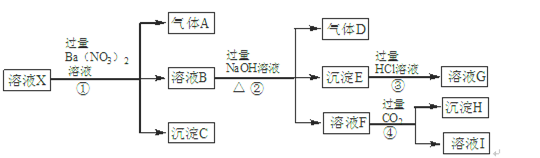
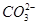 £»¢ß
£»¢ß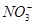 ÖŠµÄ¼øÖÖĄė×Ó£¬ĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČėNaOHČÜŅŗÖĮ¹żĮæ£¬Éś³É³ĮµķµÄÖŹĮæČēĶ¼ĖłŹ¾”£ŌņøĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠµÄĄė×ÓŹĒ
ÖŠµÄ¼øÖÖĄė×Ó£¬ĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČėNaOHČÜŅŗÖĮ¹żĮæ£¬Éś³É³ĮµķµÄÖŹĮæČēĶ¼ĖłŹ¾”£ŌņøĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠµÄĄė×ÓŹĒ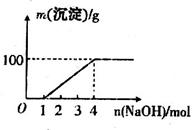
| A£®¢Ś¢Ż¢ß | B£®¢Ś¢Ż¢Ž | C£®¢Ū¢Ż¢ß | D£®¢Ł¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®x=10Ź±£¬ČÜŅŗÖŠÓŠNH4+”¢Al3+”¢SO42-£¬ĒŅc(SO42-)>c(NH4+)>c(Al3+) |
| B£®x=20Ź±ČÜŅŗµ¼µēÄÜĮ¦±Čx£½15Ź±ČÜŅŗµ¼µēÄÜĮ¦Ēæ |
| C£®x=25Ź±£¬ČÜŅŗÖŠÖ÷ŅŖÓŠBa2+”¢AlO2-£¬c(Ba2+)>c(AlO2-) |
| D£®x=30Ź±µÄĄė×Ó·½³ĢŹ½£ŗNH4++Al3++2SO42-+2Ba2++5OH-”śAlO2-+2BaSO4”ż+NH3?H2O+2H2O |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com