


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

| A£®M½Óøŗ¼«£¬N½ÓÕż¼«£¬µ±Į½¼«²śÉśĘųĢå×ÜĮæĪŖ22.4 L(±ź×¼×“æö)Ź±£¬Éś³É1 mol NaOH |
| B£®M½Óøŗ¼«£¬N½ÓÕż¼«£¬ŌŚČÜŅŗÖŠµĪČĖ·ÓĢŖŹŌŅŗ£¬Cµē¼«ÖÜĪ§ČÜŅŗ±äŗģ |
| C£®M½Óøŗ¼«£¬N½ÓÕż¼«£¬Čō°ŃÉÕ±ÖŠČÜŅŗ»»³É1 L CuSO4ČÜŅŗ£¬·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬ÉÕ±ÖŠ²śÉśĄ¶É«³Įµķ |
| D£®M½ÓµēŌ“Õż¼«£¬N½ÓµēŌ“øŗ¼«£¬½«Cµē¼«»»³ÉCuµē¼«£¬µē½āÖŹČÜŅŗ»»³ÉCuSO4ČÜŅŗ£¬ŌņæÉŹµĻÖŌŚĢśÉĻ¶ĘĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
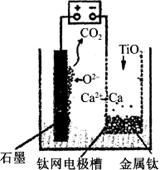
| A£®øƵē³Ų¹¤×÷¹ż³ĢÖŠO2£ĻņŅõ¼«ŅĘ¶Æ |
| B£®Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖC£«2O2££4e£===CO2”ü |
| C£®ČōÓĆĒ¦Šīµē³Ų×÷øĆ×°ÖĆµÄ¹©µēµēŌ“£¬”°£”±½ÓĻßÖłÓ¦Į¬½ÓPbO2µē¼« |
| D£®ŌŚÖʱø½šŹōīŃĒ°ŗó£¬ÕūĢ××°ÖĆÖŠCaOµÄ×ÜĮæ¼õÉŁ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®5.60L | B£®6.72L | C£®4.48L | D£®3.36L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®b<a<7 |
| B£®Ņõ¼«µē¼«·“Ó¦Ź½ĪŖ£ŗCu2++2e-="Cu" |
| C£®ĻņČÜŅŗÖŠ¼ÓČĖ9.8x(10-b-10-a)gCu(OH)2æÉŹ¹ČÜŅŗ»Öø“µ½µē½āĒ°µÄÅØ¶Č |
| D£®Ńō¼«²śÉśµÄĘųĢåŹĒO2£¬ĘäĢå»ż(±ź×¼×“æöĻĀ)ĪŖ1.12x(10-b-10-a)L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
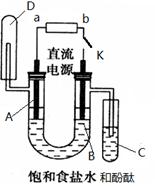
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
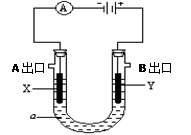
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®3.36L | B£®6.72L | C£®5.60L | D£®2.80L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®0.64 g | B£®1.28 g |
| C£®2.56 g | D£®5.12 g |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com