






| �ζ����� | ������Һ �����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.20 | 20.20 |
 H2SO3��2H2SO3��O2��2H2SO4 ��2�֣�
H2SO3��2H2SO3��O2��2H2SO4 ��2�֣� 2SO3��SO3��H2O��H2SO4 ��2SO2��O2��2H2O��2H2SO4��
2SO3��SO3��H2O��H2SO4 ��2SO2��O2��2H2O��2H2SO4��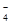 ��5SO
��5SO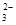 ��6H����2Mn2����5SO
��6H����2Mn2����5SO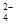 ��3H2O�� ��2�֣�
��3H2O�� ��2�֣� H2SO3��2H2SO3��O2��2H2SO4
H2SO3��2H2SO3��O2��2H2SO4 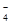 ��5SO
��5SO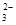 ��6H����2Mn2����5SO
��6H����2Mn2����5SO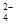 ��3H2O��
��3H2O��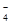 ��5SO
��5SO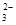 ������Ʒ���������Ƶ�����Ϊ0.02L��0.01000 mol��L��1��2.5��10��126g/mol=0.63g��������Ʒ���������Ƶ�����������0.63g/1.0000g��100%=63.00%��
������Ʒ���������Ƶ�����Ϊ0.02L��0.01000 mol��L��1��2.5��10��126g/mol=0.63g��������Ʒ���������Ƶ�����������0.63g/1.0000g��100%=63.00%��

 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| 1 |
| 2 |
| A��1.1mol?L-1 | B��1.5mol?L-1 | C��1.2mol?L-1 | D��1.0mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ˮ��Ӧ��Na+H2O��Na++OH��+H2�� |
| B������ͭ��Һ������������Һ��Ӧ��Ba2+ + SO42�� =BaSO4�� |
| C����ˮ����������Һ��Ӧ��3OH��+ Al3+ ��Al(OH)3�� |
| D����������������������Һ��Ӧ SiO2+2OH����SiO32��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Al2(SO4)3��Һ�μӰ�ˮ������ɫ��״������ Al3����3OH��= Al(OH)3�� |
B��90 ��ʱ����ô�ˮ��c(H��)��c(OH��) =3.8��10��13�� H2O(l) H��(aq)��OH��(aq)?H��0 H��(aq)��OH��(aq)?H��0 |
| C��FeCl3��Һ��ͨ��SO2����Һ��ɫ��ȥ��2Fe3����SO2 ��2H2O = 2Fe2����SO42���� 4H�� |
D��̼������Һ�����̪��죺CO32���� 2H2O  H2CO3��2OH�� H2CO3��2OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����NH4Al(SO4)2��Һ�е���Ba(OH)2ǡ��ʹSO42����Ӧ��ȫ�� NH4++2Ba2++4OH��+Al3++2SO42��= 2BaSO4��+Al(OH)3��+ NH3.H2O |
| B�������͡�84������Һ��ϣ�2H++Cl-+ClO-=Cl2��+H2O |
| C����������Һ�м���������Ba(OH)2��Һ�� 2Al3++3SO42-+3Ba2++6OH- = 3BaSO4��+2Al(OH)3�� |
| D���ڸ���NH4Fe(SO4)2��Һ����μ���Ba(OH)2��Һ�����ܷ��������ӷ���ʽ�ǣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.5mol/L��NaHSO3��Һ��1.0mol/L��NaClO��Һ�������ϣ�HSO3����ClO����SO42����Cl����H�� |
| B��С�մ���Һ��ƫ��������Һ��ϣ�HCO3����AlO2����H2O��Al(OH)3��+CO32�� |
| C����84������Һ����Ҫ�ɷ�ΪNaClO��¶���ڿ����б��ʣ�2ClO����CO2��H2O��CO32����2HClO |
| D���ڡ�84������Һ�еμ�FeSO4��Һ��2Fe2����ClO����H2O��2Fe3����Cl����2OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������۵⻯����Һ�еμ�ϡ���ᣬ�ڿ����з���һ��ʱ�����Һ������4H����4I����O2��2I2��2H2O |
B����K2Cr2O7��Һ�еμ�����Ũ���ᣬ��Һ��Ϊ��ɫ�� Cr2O72��(��ɫ)��H2O 2CrO42��(��ɫ)��2H�� 2CrO42��(��ɫ)��2H�� |
| C��0.01 mol��L-1NH4Al(SO4)2��Һ��0.02 mol��L-1Ba(OH )2��Һ���������а�ɫ�������ɣ� Al3����2SO42����2Ba2+��4OH����2BaSO4����AlO2����2H2O |
D����ͭ�缫��ⱥ��ʳ��ˮ��2Cl����2H2O Cl2����H2����2OH�� Cl2����H2����2OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A������ͨ��ˮ�У�Cl2 + H2O  2H+ + Cl��+ClO�� 2H+ + Cl��+ClO�� |
| B���������������м���HI��Һ��Fe(OH)3 + 3H+ = Fe3+ + 3H2O |
| C��NaAlO2��Һ��ͨ�����CO2��2AlO2��+ CO2 + 3H2O = 2Al(OH)3��+ CO32�� |
| D����ϡ�����ȥ�Թ��ڱ�����3Ag+ 4H+ + NO3�� = 3Ag+ + NO�� +2H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com