
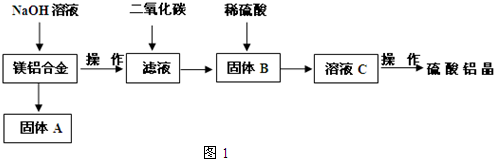
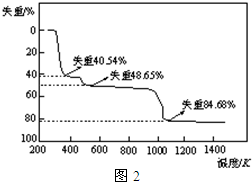
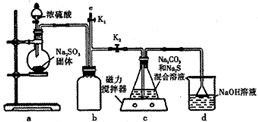
 £Ø5£©Č”ÉĻŹöĮņĖįĀĮ¾§Ģå½ųŠŠČČÖŲ·ÖĪö£¬ĘäČČ·Ö½āÖ÷ŅŖ·ÖĪŖČżøö½×¶Ī£ŗ323K-523K£¬553K-687K£¬1043KŅŌÉĻ²»ŌŁŹ§ÖŲ£¬ĘäČČ·Ö½āµÄTGĒśĻß¼ūĶ¼2£¬ŅŃÖŖ£ŗŹ§ÖŲ%=$\frac{¼ÓČČ¼õÉŁµÄÖŹĮæ}{Ō¾§ĢåѳʷµÄ×ÜÖŹĮæ}$”Į100%£®øł¾ŻĶ¼Ź¾Źż¾Ż¼ĘĖćČ·¶ØĆæ²½·Ö½āµÄ²śĪļ£¬Š“³öµŚŅ»½×¶Ī·Ö½ā²śĪļµÄ»ÆѧŹ½Al2£ØSO4£©3.3H2O£¬µŚČż½×¶Ī·“Ó¦»Æѧ·½³ĢŹ½Al2£ØSO4£©3$\frac{\underline{\;\;”÷\;\;}}{\;}$Al2O3+3SO3”ü£®
£Ø5£©Č”ÉĻŹöĮņĖįĀĮ¾§Ģå½ųŠŠČČÖŲ·ÖĪö£¬ĘäČČ·Ö½āÖ÷ŅŖ·ÖĪŖČżøö½×¶Ī£ŗ323K-523K£¬553K-687K£¬1043KŅŌÉĻ²»ŌŁŹ§ÖŲ£¬ĘäČČ·Ö½āµÄTGĒśĻß¼ūĶ¼2£¬ŅŃÖŖ£ŗŹ§ÖŲ%=$\frac{¼ÓČČ¼õÉŁµÄÖŹĮæ}{Ō¾§ĢåѳʷµÄ×ÜÖŹĮæ}$”Į100%£®øł¾ŻĶ¼Ź¾Źż¾Ż¼ĘĖćČ·¶ØĆæ²½·Ö½āµÄ²śĪļ£¬Š“³öµŚŅ»½×¶Ī·Ö½ā²śĪļµÄ»ÆѧŹ½Al2£ØSO4£©3.3H2O£¬µŚČż½×¶Ī·“Ó¦»Æѧ·½³ĢŹ½Al2£ØSO4£©3$\frac{\underline{\;\;”÷\;\;}}{\;}$Al2O3+3SO3”ü£® ·ÖĪö ĻņĆ¾ĀĮŗĻ½šÖŠ¼ÓČė×ćĮæĒāŃõ»ÆÄĘČÜŅŗ£¬·¢Éś·“Ó¦£ŗ2Al+2NaOH+2H2OØT2NaAlO2+3H2”ü£¬Ć¾²»·“Ó¦£¬¹ĢĢåAĪŖMg£¬²ÉÓĆ¹żĀĖµÄ·½·Ø½ųŠŠ·ÖĄė£¬ĻņĀĖŅŗÖŠĶØČė¶žŃõ»ÆĢ¼£¬·¢Éś·“Ó¦£ŗNaAlO2+CO2+2H2OØTAl£ØOH£©3”ż+NaHCO3£¬ŌŁĶعż¹żĮæ½ųŠŠ·ÖĄė£¬¹ĢĢåBĪŖĒāŃõ»ÆĀĮ£¬ĒāŃõ»ÆĀĮÓėĮņĖį·“Ó¦µĆµ½ĮņĖįĀĮČÜŅŗ£¬ŌŁ¾¹żÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢Ļ“µÓ”¢øÉŌļµĆµ½ĮņĖįĀĮ¾§Ģ壻
£Ø1£©AlŗĶĒāŃõ»ÆÄĘČÜŅŗÉś³ÉæÉČÜŠŌµÄĘ«ĀĮĖįÄĘ£¬Ć¾²»·“Ó¦£»
£Ø2£©“ÓČÜŅŗÖŠ»ńµĆ¾§Ģ壬ŠčŅŖ¾¹żÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµČ²Ł×÷£»
£Ø3£©ÓĆŅŅ“¼Ļ“µÓ£¬æÉŅŌ¼õÉŁ¾§ĢåµÄČܽā£¬ÓŠĄūÓŚ¾§ĢåµÄøÉŌļ£»
£Ø4£©AlµÄÖŹĮæĪŖ9g-4.95g=4.05g£¬ÉčĮņĖįĀĮ¾§Ģå»ÆѧŹ½ĪŖ£ŗAl2£ØSO4£©3£®nH2O£¬øł¾ŻAlŌŖĖŲŹŲŗć¼ĘĖćĮņĖįĀĮ¾§ĢåµÄĪļÖŹµÄĮ棬ŌŁ¼ĘĖćĮņĖįĀĮ¾§ĢåµÄĻą¶Ō·Ö×ÓÖŹĮ棬½ų¶ų¼ĘĖćnµÄÖµ£¬Č·¶Ø»ÆѧŹ½£»
£Ø5£©øł¾Ż£Ø4£©ÖŠ¼ĘĖćæÉÖŖ£¬¾§ĢåÖŠ½į¾§Ė®µÄÖŹĮæ·ÖŹż£¬µĶĪĀ¼ÓČČ£¬Ź×ĻČŹ§Č„½į¾§Ė®£¬øßĪĀĻĀ£¬×īÖÕĮņĖįĀĮ·Ö½ā£¬øł¾ŻŹ§ÖŲ%¼ĘĖćÅŠ¶Ļø÷½×¶Ī·Ö½ā²śĪļ£¬ŌŁŹéŠ“»Æѧ·½³ĢŹ½£®
½ā“š ½ā£ŗĻņĆ¾ĀĮŗĻ½šÖŠ¼ÓČė×ćĮæĒāŃõ»ÆÄĘČÜŅŗ£¬·¢Éś·“Ó¦£ŗ2Al+2NaOH+2H2OØT2NaAlO2+3H2”ü£¬Ć¾²»·“Ó¦£¬¹ĢĢåAĪŖMg£¬²ÉÓĆ¹żĀĖµÄ·½·Ø½ųŠŠ·ÖĄė£¬ĻņĀĖŅŗÖŠĶØČė¶žŃõ»ÆĢ¼£¬·¢Éś·“Ó¦£ŗNaAlO2+CO2+2H2OØTAl£ØOH£©3”ż+NaHCO3£¬ŌŁĶعż¹żĮæ½ųŠŠ·ÖĄė£¬¹ĢĢåBĪŖĒāŃõ»ÆĀĮ£¬ĒāŃõ»ÆĀĮÓėĮņĖį·“Ó¦µĆµ½ĮņĖįĀĮČÜŅŗ£¬ŌŁ¾¹żÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢Ļ“µÓ”¢øÉŌļµĆµ½ĮņĖįĀĮ¾§Ģ壻
£Ø1£©AlŗĶĒāŃõ»ÆÄĘČÜŅŗÉś³ÉæÉČÜŠŌµÄĘ«ĀĮĖįÄĘÓėĒāĘų£¬Ć¾²»·“Ó¦£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗ2Al+2NaOH+2H2OØT2NaAlO2+3H2”ü£¬ÓÉÉĻŹö·ÖĪöæÉÖŖ£¬¹ĢĢåBĪŖAl£ØOH£©3£¬
¹Ź“š°øĪŖ£ŗ2Al+2NaOH+2H2OØT2NaAlO2+3H2”ü£»Al£ØOH£©3£»
£Ø2£©“ÓČÜŅŗÖŠ»ńµĆ¾§Ģ壬ŠčŅŖ¾¹żÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµČ²Ł×÷£¬
¹Ź“š°øĪŖ£ŗĄäČ“½į¾§”¢¹żĀĖ£»
£Ø3£©ÓĆŅŅ“¼Ļ“µÓ£¬æÉŅŌ¼õÉŁ¾§ĢåµÄČܽā£¬ÓŠĄūÓŚ¾§ĢåµÄøÉŌļ£¬
¹Ź“š°øĪŖ£ŗæÉŅŌ¼õÉŁ¾§ĢåµÄČܽā£¬ÓŠĄūÓŚ¾§ĢåµÄøÉŌļ£»
£Ø4£©AlµÄÖŹĮæĪŖ9g-4.95g=4.05g£¬ĘäĪļÖŹµÄĮæĪŖ$\frac{4.05g}{27g/mol}$=0.15mol£¬ÉčĮņĖįĀĮ¾§Ģå»ÆѧŹ½ĪŖ£ŗAl2£ØSO4£©3£®nH2O£¬øł¾ŻAlŌŖĖŲŹŲŗć£¬ĮņĖįĀĮ¾§ĢåµÄĪļÖŹµÄĮæĪŖ$\frac{0.15mol}{2}$=0.075mol£¬¹ŹĮņĖįĀĮ¾§ĢåµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ$\frac{49.95}{0.075}$=666£¬Ōņ54+96”Į3+18n=666£¬½āµĆn=18£¬¹ŹøĆĮņĖįĀĮ¾§ĢåµÄ»ÆѧŹ½ĪŖ£ŗAl2£ØSO4£©3.18H2O£¬
¹Ź“š°øĪŖ£ŗAl2£ØSO4£©3.18H2O£»
£Ø5£©¾§ĢåÖŠ½į¾§Ė®µÄŗ¬ĮæĪŖ$\frac{18”Į18}{666}$=48.65%£¬¹ŹµŚ¶ž½×¶ĪĶźČ«Ź§Č„½į¾§Ė®£¬µĆµ½ĪļÖŹĪŖAl2£ØSO4£©3£¬µŚŅ»½×¶ĪŹ§Č„²æ·Ö½į¾§Ė®£¬Ź§Č„½į¾§Ė®ŹżÄæĪŖ$\frac{666”Į40.54%}{18}$=15£¬¹ŹµŚŅ»½×¶ĪµĆµ½µÄĪļÖŹĪŖAl2£ØSO4£©3.3H2O£¬
µŚČż½×¶ĪŹ£ÓąĪļÖŹµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ666”Į£Ø1-84.68%£©=102£¬Ó¦ŹĒAl2O3£¬¹ŹĮņĖįĀĮ·“Ӧɜ³ÉŃõ»ÆĀĮÓėČżŃõ»ÆĮņ£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗAl2£ØSO4£©3$\frac{\underline{\;\;”÷\;\;}}{\;}$Al2O3+3SO3”ü£¬
¹Ź“š°øĪŖ£ŗAl2£ØSO4£©3.3H2O£»Al2£ØSO4£©3$\frac{\underline{\;\;”÷\;\;}}{\;}$Al2O3+3SO3”ü£®
µćĘĄ ±¾Ģāæ¼²éŹµŃéÖʱø·½°ø”¢ĪļÖŹ·ÖĄėĢį“攢·½°ø·ÖĪöĘĄ¼Ū”¢ĪļÖŹ×é³É²ā¶ØµČ£¬ŹĒ¶Ōѧɜ×ŪŗĻÄÜĮ¦µÄ漲飬עŅā£Ø5£©ÖŠ³ä·ÖĄūÓĆĮņĖįĀĮ¾§ĢåĻą¶Ō·Ö×ÓÖŹĮæ½ųŠŠ¼ĘĖć½ā“š£¬ÄѶČÖŠµČ£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
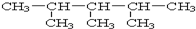 £ŗ2£¬3£¬4-Čż¼×»łĪģĶ飮
£ŗ2£¬3£¬4-Čż¼×»łĪģĶ飮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Ģģ½ņøŪ”°8.12”±±¬ÕØŹĀ¹ŹÖŠ£¬Ņņ±¬Õسå»÷µ¼ÖĀĒč»ÆÄĘŠ¹Ā©£¬æÉŅŌĶعżÅēČ÷Ė«ŃõĖ®»ņĮņ“śĮņĖįÄĘČÜŅŗĄ““¦Ąķ£¬ŅŌ¼õĒįĪŪČ¾£®
Ģģ½ņøŪ”°8.12”±±¬ÕØŹĀ¹ŹÖŠ£¬Ņņ±¬Õسå»÷µ¼ÖĀĒč»ÆÄĘŠ¹Ā©£¬æÉŅŌĶعżÅēČ÷Ė«ŃõĖ®»ņĮņ“śĮņĖįÄĘČÜŅŗĄ““¦Ąķ£¬ŅŌ¼õĒįĪŪČ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé²Ł×÷ | ŹµŃéĻÖĻó | ½įĀŪ |
| A | ½«”°84”±Ļū¶¾Ņŗ£Øŗ¬NaClO£©µĪČėĘ·ŗģČÜŅŗÖŠ£¬ĶŹÉ«»ŗĀż£¬ČōĶ¬Ź±¼ÓČėŹ³“× | ŗģÉ«ŗÜæģĶŹĪŖĪŽÉ« | ĖęČÜŅŗpH¼õŠ”£¬NaClOµÄŃõ»ÆÄÜĮ¦ŌöĒæ |
| B | ĻņijĀČ»ÆŃĒĢśČÜŅŗÖŠ¼ÓČėNa2O2·ŪÄ© | ³öĻÖŗģŗÖÉ«³Įµķ | ĖµĆ÷ŌĀČ»ÆŃĒĢśŅŃŃõ»Æ±äÖŹ |
| C | ±½·ÓŗĶĖ®µÄ×ĒŅŗÖŠ£¬¼ÓNa2CO3ČÜŅŗ | ČÜŅŗ±ä³ĪĒå | ±½·ÓµÄĖįŠŌ±ČĢ¼ĖįĒæ |
| D | ĻņČÜŅŗXÖŠ¼ÓČėNaHCO3·ŪÄ© | ²śÉśĪŽÉ«ĘųĢå | ČÜŅŗXµÄČÜÖŹŅ»¶ØŹōÓŚĖį |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗćČŻĆܱÕČŻĘ÷ÖŠ½ųŠŠµÄ·“Ó¦3A£Øg£©?B£Øg£©+c£Øg£©£¬ŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬ŌŁ³äČėŅ»¶ØĮæµÄAĘųĢ壬AµÄ×Ŗ»ÆĀŹ½«Ōö“ó | |
| B£® | ¶ŌÓŚæÉÄę·“Ó¦N2£Øg£©+3H2£Øg£©?2NH3£Øg£©£¬Ōö“óµŖĘųÅضČæÉŌö¼Ó»ī»Æ·Ö×Ó°Ł·ÖŹż£¬“Ó¶ųŹ¹·“Ó¦ĖŁĀŹŌö¼Ó | |
| C£® | ½«FeCl3ČÜŅŗŗĶNaAlO2ČÜŅŗĻą»ģ£¬ĖłµĆ¹ĢĢå²śĪļFe£ØOH£©3 | |
| D£® | ·“Ó¦NH3£Øg£©+HCl£Øg£©?NH4Cl£Øs£©”÷H£¼OŌŚČĪŗĪĢõ¼žĻĀ¾łÄÜ×Ō·¢½ųŠŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
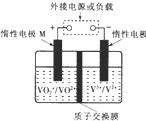 Č«·°ŅŗĮ÷“¢Äܵē³ŲĄūÓĆ²»Ķ¬¼ŪĢ¬Ąė×Ó¶ŌµÄŃõ»Æ»¹Ō·“Ó¦Ą“ŹµĻÖ»ÆѧÄÜŗĶµēÄܵÄĻą»„×Ŗ»Æ£¬³äµēŹ±£¬¶čŠŌµē¼«M”¢N·Ö±šĮ¬½ÓµēŌ“µÄÕż¼«ŗĶøŗ¼«£®µē³Ų¹¤×÷ŌĄķČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©
Č«·°ŅŗĮ÷“¢Äܵē³ŲĄūÓĆ²»Ķ¬¼ŪĢ¬Ąė×Ó¶ŌµÄŃõ»Æ»¹Ō·“Ó¦Ą“ŹµĻÖ»ÆѧÄÜŗĶµēÄܵÄĻą»„×Ŗ»Æ£¬³äµēŹ±£¬¶čŠŌµē¼«M”¢N·Ö±šĮ¬½ÓµēŌ“µÄÕż¼«ŗĶøŗ¼«£®µē³Ų¹¤×÷ŌĄķČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | ³äµē¹ż³ĢÖŠ£¬Nµē¼«ø½½üĖįŠŌ¼õČõ | |
| B£® | ³äµē¹ż³ĢÖŠ£¬Nµē¼«ÉĻV3+±»»¹ŌĪŖV2+ | |
| C£® | ·Åµē¹ż³ĢÖŠ£¬H+ÓÉNµē¼«ĻņMµē¼«ŅĘ¶Æ | |
| D£® | ·Åµē¹ż³ĢÖŠ£¬Mµē¼«·“Ó¦ĪŖV02++2H++e-ØTV02++H20 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com