
����Ŀ��CuSO4��һ����Ҫ�Ļ���ԭ�ϣ����й��Ʊ�;����������ͼ��ʾ������˵������ȷ���ǣ� ��
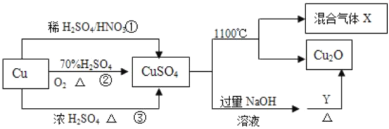
A. �����;���٢���;�������õ���������ɫ��ѧ˼��
B. ;���������ķ�Ӧ������3Cu+2HNO3+3H2SO4=3CuSO4+2NO![]() +4H2O
+4H2O
C. 1mol CuSO4��1100�����û������XΪSO3��O2������O2Ϊ0.5mo1
D. ��CuSO4��Һ����Ũ������ȴ�ᾧ�����Ƶõ�������(CuSO4��5H2O)
���𰸡�C
��������
A.;����������Ⱦ����������NO��;����������Ⱦ����������SO2����;������������Ⱦ���������ʣ����õ���������ɫ��ѧ˼�룬A��ȷ��
B.;�������������ӷ�ӦΪ3Cu+8H++2NO3-=3Cu2++2NO![]() +4H2O����Ӧ������3Cu+2HNO3+3H2SO4=3CuSO4+2NO
+4H2O����Ӧ������3Cu+2HNO3+3H2SO4=3CuSO4+2NO![]() +4H2O��B��ȷ��
+4H2O��B��ȷ��
C.1molCuSO4��1100�����û������XΪSO3��SO2��O2��C����
D. ��CuSO4��Һ����Ũ������ȴ�ᾧ�����ˣ����Ƶõ�������(CuSO4��5H2O)��D��ȷ��
��ΪC��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���� NA ���������ӵ�����������˵����ȷ����
A.��1mol��C2H5OHˮ��Һ����ԭ������Ϊ6NA
B.��״���£����Ϊ22.4L��CO2��NO������壬��ԭ����һ��С��NA
C.��״���£�11.2L SO3�����ķ�����Ϊ0.5NA
D.��1L 2mol��L-1��FeCl3��Һ�Ƴɽ���������к��е���������������Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С�鰴����·�ߺϳ���Ҫ�л�����м���J

(1)����˵������ȷ����______��
A.������A��B�ķ�Ӧ����Ϊ�ӳɷ�Ӧ
B.������F�ܷ���ȡ�����ӳɡ���ȥ�ȷ�Ӧ
C.������G���������
D.������J�ķ�����C16H22N2O3
(2)������E�Ľṹ��ʽ��_____��
(3)C+H��I�Ļ�ѧ����ʽ��______��
(4)д��������Xͬʱ��������������ͬ���칹��Ľṹ��ʽ______��IR�������������в�����-NH2
(5)��CH2=CH2��HCHOΪԭ���Ʊ�C(CH2OH)4������ƺϳ�·�ߣ�������ͼ��ʾ�����Լ���ѡ��_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С��������CuO��NH3��Ӧ���о�NH3��ij�����ʲ��ⶨ����ɣ����������ʵ��װ��(�г�װ��δ����)����ʵ�顣��ش��������⣺
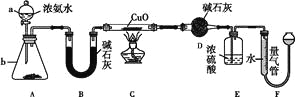
(1)����a������Ϊ____������b�п�ѡ����Լ�Ϊ____��
(2)ʵ������,����װ��A��������ȡ����ɫ������____(����ĸ)��
A��Cl2 | B��O2 | C��CO2 | D��NO2 |
(3)ʵ���й۲쵽װ��C�к�ɫCuO��ĩ��Ϊ��ɫ���壬����������ɫ��ζ�������������������֤��NH3����____��,д����Ӧ�Ļ�ѧ����ʽ:_______________________��
(4)Eװ����Ũ�����������_____________________________________��
(5)��ȡ�������ǰ��Ӧ��װ��F���еIJ�����____________________________��
(6)ʵ����ϣ�����ø����D����m g��װ��F�����������Ϊn L��������ɱ�״�����������е������ԭ�Ӹ�����Ϊ____���ú�m��n��ĸ�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���ɳ���Ԫ����ɵ�һЩ���ʼ��仯����֮���ת����ϵͼ���������ʾ�йص�һ�ַ�Ӧ�����������������Ѿ���ȥ��������A��B��D�ڳ����¾�Ϊ��ɫ��ζ�����壬C����ʹʪ��ĺ�ɫʯ����ֽ���������壬M���������ɫҺ�塣
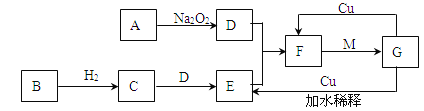
��1��д��C��E�Ļ�ѧ����ʽ�� ��
��2��ʵ���ҳ��ü������ֹ�������ķ����Ʊ�����C���仯ѧ����ʽΪ�� ������C���� ����д�Լ����ƣ���
��3��E��������D����ʱ����۲쵽 ����������ˮ���ռ�F�������ռ���ƿ���ռ���������Ϊ ����д���ʵĻ�ѧʽ����
��4��д��A��D�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��þ���Ͻ�Ͷ�뵽20mL1mol/L�������У����Ͻ���ȫ�ܽ������Һ�����1mol/L��NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�����mL���仯�Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ� ��

A. þ�����γɺϽ��Ӳ����ǿ���۵㽵��B. ab�ο��Լ�����Ͻ���Mg�ĺ���
C. cֵԽ�Ͻ���Al�ĺ���Խ��D. ����NaOH��Һ��ֻ������4�����ӷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(AlN)��һ���������ǽ������ϡ�ijAlN��Ʒ������Al2O3���ʣ�Ϊ�ⶨAlN�����������������������ʵ�鷽����
��1����֪AlN��NaOH��Ӧ�õ�һ���κ�һ�ּ������壬��ѧ��Ӧ����ʽ��____
������1��ȡһ��������Ʒ����ͼ1װ�òⶨ��Ʒ��AlN�������������г���������ȥ����
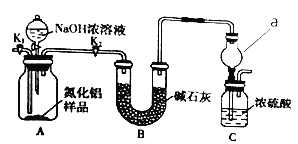

��2��ͼ1������a��������___
��3���������ʵ�鲽�裺��װ��ʵ��װ�ã����ȼ��װ�������ԣ��ټ���ʵ��ҩƷ���ر�K1����K2�ͷ�Һ©������������NaOHŨ��Һ�����ٲ������壬��K1��ͨ�뵪��һ��ʱ�䣬�ⶨCװ���ڷ�Ӧǰ��������仯��ͨ�뵪����Ŀ����____
��4������װ�ô���ȱ�ݣ����²ⶨ���ƫ�ߣ�������Ľ����____
������2����ͼ2װ�òⶨmg��Ʒ��AlN���������������ּг�װ�ü���ȥ����
��5��Ϊ�ⶨ������������������װ���е�XҺ�������___������ĸ��ţ���
a��CCl4 b��H2O c������NH4Cl��Һ d����
��6����mg��Ʒ��ȫ��Ӧ�����������������ΪVmL����״��������AlN����������Ϊ___������b�������õ��ɼм�ס���������������䣬�����ղⶨ�Ľ��___������ƫ������ƫС��������Ӱ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ʵ��ԭ����װ�á���������۵��������������
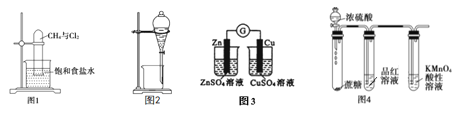
A. ͼ1��ʾװ�ÿ�ʵ�ּ����������ڹ��������µ�ȡ����Ӧ
B. ͼ2��ʾװ�ÿɷ���CH3COONa��Һ��CH3COOC2H5�Ļ��Һ
C. ͼ3��ԭ���װ�ã������Եĵ���
D. ͼ4��ʾװ�ÿ�˵��ŨH2SO4������ˮ�ԡ�ǿ�����ԣ�SO2����Ư���ԡ���ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C���ֳ����Ľ������ʣ�A�ڿ�����ȼ�����ɵ���ɫ����ף�B�ڿ����м����ۻ��������䣻C��һ����������ˮ������Ӧ����H2��һ�ֺ�ɫ���塣
�����������Ϣ�ش��������⣺
(1)д���������ʻ�ѧʽ��A_________B_________C_________��_________
(2)��Ҫ��д������ʽ��
��A�ڿ�����ȼ�յĻ�ѧ����ʽ��___________________________________________
��B������������Һ��Ӧ�����ӷ���ʽ��_____________________________________
��C��ˮ������Ӧ�Ļ�ѧ����ʽ��___________________________________________
�ܼ���ˮ��Ӧ�Ļ�ѧ����ʽ��_______________________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com