
����Ŀ���ϳɰ��������ѧ������չʷ�ϵ�һ���ش�ͻ�ƣ��о�����Һ����һ�����õĴ������ʡ�
(1) �����ֽⷴӦ���Ȼ�ѧ����ʽ���£�2NH3(g) ![]() N2(g)��3H2(g)����H
N2(g)��3H2(g)����H
����N��N����H��H����N��H���ļ��ֱܷ����a��b��c(��λ��kJ��mol1)��������Ӧ����H��________kJ��mol1��
(2) �о��������������ɼ��ٰ����ķֽ⡣�±�Ϊij�¶��µ������IJ�ͬ�����ֱ����Ũ�Ȱ����ֽ����������ij�ʼ����(mmol��min1)��
���� | Ru | Rh | Ni | Pt | Pd | Fe |
��ʼ���� | 7.9 | 4.0 | 3.0 | 2.2 | 1.8 | 0.5 |
�ٲ�ͬ���������£������ֽⷴӦ���������________(��д�����Ļ�ѧʽ)��
���¶�ΪT����һ����̶����ܱ������м���2 mol NH3����ʱѹǿΪP0����Ru�������ֽ⣬��ƽ��ʱ�����ֽ��ת����Ϊ50%������¶��·�Ӧ2NH3(g) ![]() N2(g)��3H2(g)��ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp��________��[��֪�������ѹ(p��)��������ѹ(p��)���������]
N2(g)��3H2(g)��ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp��________��[��֪�������ѹ(p��)��������ѹ(p��)���������]
(3) ���ںϳɰ����յ����⣬������ȷ����________��
A���ϳɰ���ҵ�����õķ�Ӧ�¶�Ϊ500�����ң�������������ԭ������
B��ʹ�ó�ʼ��Ӧ���ʸ���Ĵ���Ru���������ƽ��ʱNH3�IJ���έ
C���ϳɰ���ҵ����10 MPa��30 MPa������ѹ��N2��H2��ת���ʲ���
D��������ˮ���µķ����ɽ��ϳɺ��������еİ�Һ��
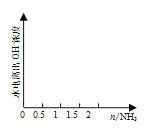
(4) ��1 L 1 mol��L1�������л���ͨ��2 mol����������ͼ�л�����Һ��ˮ�������OHŨ���氱��ͨ��仯������ͼ��______________________________
(5) �绯ѧ��Ҳ�ɺϳɰ�����ͼ���õ��¹������ӵ�����Ϊ����ʣ���PtC3N4�������������H2(g)��N2(g)�ϳ�NH3��ԭ��ʾ��ͼ��
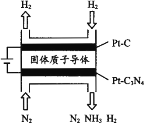
��PtC3N4�缫��Ӧ����NH3�ĵ缫��Ӧʽ________��
��ʵ���о�����������ӵ�ѹ����һ��ֵ�Ժ������������а���������������ŵ�ѹ���������С�����������ԭ��________��
���𰸡�6c��a��3b Fe ![]() BC
BC 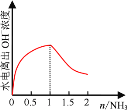 N2��6e��6H+=2NH3 ����һ����ѹ�Ժ�H+�õ��ӱ��H2���������ʱȵ�����
N2��6e��6H+=2NH3 ����һ����ѹ�Ժ�H+�õ��ӱ��H2���������ʱȵ�����
��������
��1��2NH3(g) ![]() N2(g)��3H2(g)�У�N��N��1����N��H��6����H-H��3������H=��Ӧ��ļ���֮����������ļ���֮��=6c��a��3b��
N2(g)��3H2(g)�У�N��N��1����N��H��6����H-H��3������H=��Ӧ��ļ���֮����������ļ���֮��=6c��a��3b��
��2�����������ɼ��ٰ����ķֽ⣬��ӦԽ����˵����Ӧ����Ļ��Խ�ݱ���֪��Fe��ʱ��Ӧ���������������ֽⷴӦ���������Fe��
��3����Ӧ2NH3(g) ![]() N2(g)��3H2(g)��ʼʱ��2 mol NH3����ʱѹǿΪP0��NH3��ת����Ϊ50%����Ӧƽ��ʱ��ϵ����1mol NH3��0.5mol N2��1.5mol H2�������ʵ���Ϊ3mol������ƽ��ʱ����ѹΪ
N2(g)��3H2(g)��ʼʱ��2 mol NH3����ʱѹǿΪP0��NH3��ת����Ϊ50%����Ӧƽ��ʱ��ϵ����1mol NH3��0.5mol N2��1.5mol H2�������ʵ���Ϊ3mol������ƽ��ʱ����ѹΪ![]() P0�������ķ�ѹΪ
P0�������ķ�ѹΪ![]() P0�������ķ�ѹΪ
P0�������ķ�ѹΪ![]() P0�������ķ�ѹΪ
P0�������ķ�ѹΪ![]() P0����Kp=
P0����Kp=![]() =
=![]() ��
��
��4��A. �ϳɰ��ķ�Ӧ�Ƿ��ȵģ����Ժϳɰ�ʱ�¶�Խ�ͣ�����ת����Խ�ߡ����¶ȹ�����Ӱ������Ļ��ԣ������¶�Ϊ500�棬����������ԭ��û�й�ϵ����A����
B. ʹ�ô������ܸı䷴Ӧ�̶ȣ��ʲ������ƽ��ʱNH3�IJ�����B��ȷ��
C. �ϳɰ���ҵ����10 MPa��30 MPa������ѹ��N2��H2��ת���ʲ��ߣ���C��ȷ��
D. ����������ˮ�����Բ��ܲ�����ˮ���µķ����ɽ��ϳɺ��������еİ�Һ������D����
��ȷ����BC��
��5���տ�ʼͨ��NH3��NH3��HCl��Ӧ�����Ȼ�泥��ٽ�ˮ�ĵ��룬��NH3��������1molʱ��HCl��Ӧ�꣬���ɰ�ˮ����������ˮ�ĵ��룬���Դ���ͼ��

��6��PtC3N4�缫���������õ��ӣ��缫����ʽΪ��N2��6e��6H+=2NH3������ӵ�ѹ����һ��ֵ�Ժ������������а���������������ŵ�ѹ���������С�����������ԭ���dz���һ����ѹ�Ժ�H+�õ��ӱ��H2���������ʱȵ����졣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ������ͼ��ʾװ���Ʊ�����������ʵ�����£�
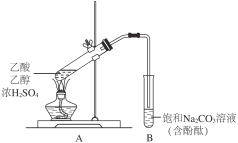
����2 mLŨH2SO4��2 mL�Ҵ����Һ�е���2 mL��������Թ�A��
��һ��ʱ����Թ�B�к�ɫ��Һ�Ϸ�������״Һ�壻
��ֹͣ���ȣ����Թ�B����״Һ���䱡���²��ɫ��Һ��ɫ��
(1)Ϊ�˼ӿ�������Ӧ���ʣ���ͬѧ��ȡ�Ĵ�ʩ��_____________��
(2)����������ת���ʣ����ɲ�ȡ�Ĵ�ʩ��_______________��
(3)�Թ�B����Һ�Ժ�ɫ��ԭ����___________�������ӷ���ʽ��ʾ����
(4)������״Һ��ijɷ���__________��
(5)���к�ɫ��ȥ��ԭ�����Ƿ�̪�������������С�֤�����Ʋ��ʵ�鷽����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ֶ�����Ԫ�������ڱ��е�λ����ͼ��ʾ��X��Y�ĺ��������֮�͵���W�ĺ��������������˵������ȷ���ǣ� ��
![]()
A.X��Y��Z����Ԫ�ص����������������
B.Y��Z�γɵļ��⻯������ȶ���ǿ
C.Y��Z�γɵļ������ӣ����߰뾶С
D.��ҵ���õ��W��Z�γɵĻ������Ʊ�����W
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��ʯ���ѽ�������Ҫ�ɷ֣����IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����Aԭ�����������ֻ�����Ʒ�ķ�Ӧ�������£����ַ�Ӧ��������ȥ����
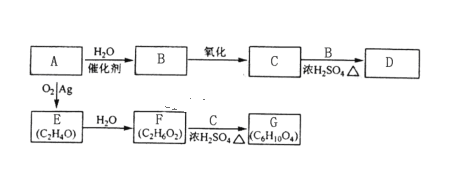
��֪����2RCHO+O2![]() 2RCOOH����R-CH��CH-OHϩ��ʽ�Ľṹ�����ȶ����ڡ���ش��������⣺
2RCOOH����R-CH��CH-OHϩ��ʽ�Ľṹ�����ȶ����ڡ���ش��������⣺
��1��A�ĵ���ʽΪ___��
��2��B��D�����еĹ��������Ʒֱ���____��____��
��3��C��B��Ӧ����D�Ļ�ѧ����ʽΪ________��
��4��E�Ľṹ��ʽΪ_____��
��5��������⣬д��E���ܵ�ͬ���칹��Ľṹ��ʽ_____��
��6��F��C��Ӧ����G�Ļ�ѧ����ʽΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮMgBr2������������ij��ȤС��ͬѧ����þм��Һ��Ϊԭ���Ʊ���ˮMgBr2�����װ����ͼ��ʾ����֪��Mg��Br2��Ӧ���ҷ��ȣ�MgBr2����ǿ��ˮ�ԡ�����˵����ȷ���ǣ� ��
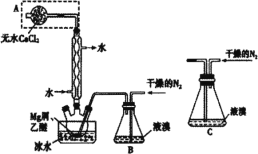
A.����������ˮ�������ڷ������
B.ʵ���п����ø���Ŀ�����������N2
C.Ϊ��ֹ��Ӧ���ھ��ң�������װ��C����װ��B
D.װ����ˮCaCl2����A�����������ջӷ�������������ֹ��Ⱦ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������к���������������������������������Ϊͬ���칹�壬����Է�������Ϊ136����������ת�����ش�������⣺
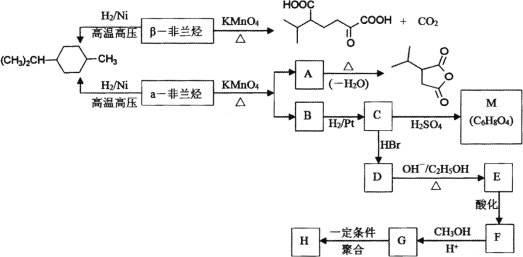
��֪��
![]()
![]()
![]() ��RCHO
��RCHO
![]()
![]()
![]() ��CO2
��CO2
2CH3COOH![]()
![]()
(1) д�����������й���������________�����������Ľṹ��ʽ________��
(2) �����й�˵����ȷ����________��
A����������������ʵ�����Br2���мӳɷ�Ӧ�����ﹲ��3��
B��C��D��E��F��Ӧ������ͬ
C���ۺ���H������ˮ
D��C��M��Ӧ�������и߷��Ӿۺ���ȸ��������
(3) д��F��G�Ļ�ѧ����ʽ________��
(4) д����������������A��ͬ���칹��________��
�ٺ���4����CH3����1 mol��ͬ���칹���ڼ���������ˮ����2 mol NaOH��
(5) �Լױ��ͱ�ϩΪ����ԭ�Ϻϳ�![]() (������ͼ��ʾ���������Լ���ѡ)________��
(������ͼ��ʾ���������Լ���ѡ)________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ�����������õ�������ѧ���ʣ����㷺Ӧ�������������С��Ӻ��ܷ���(��CoO��Co2O3������Al��Li��)����ȡ��CoCl2��6H2O��������ͼ��ʾ��
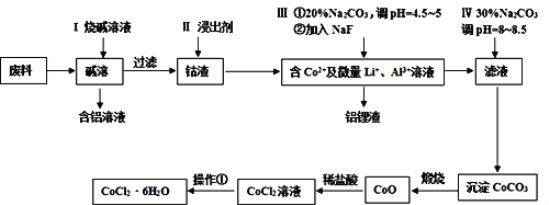
��ش��������⣺
(1)����I�з�����Ӧ�Ļ�ѧ����ʽ_______��
(2)��֪Co2O3����ǿ�����ԣ�������II�н�����Ϊ���ᣬ��ɵĺ��_______��������II�н�����Ϊ���ᣬд��Co2O3�����ᷴӦ�Ļ�ѧ����ʽ______��
(3)������Тٵ�Ŀ���dz�ȥAl3+���ڵ�Ŀ���dz�ȥLi+��д����ȥLi+�����ӷ���ʽ_______��
(4)����ʵ��������CoCO3������Ĺ��������������ƾ��ƺͲ������⣬����______��______(����������)��
(5)����������HCl��Χ�н��еģ��䲽����______��_______�����ˡ�ϴ�ӡ������75%�ƾ���������ˮϴ�ӣ����ŵ���______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������[CH3CH(OH)COO]2Fe3H2O��Mr=288����һ��ʳ�õIJ�����������Ч���������ã�������ˮ�������������Ҵ��������ֽ⣬��ͨ��������̼��������Ӧ�Ƶá�
CH3CH(OH)COOH+FeCO3+2H2O=[CH3CH(OH)COO]2Fe3H2O+CO2��
FeCO3������ˮ���ױ�������4FeCO3+6H2O+O2=4Fe(OH)3+4CO2
��.�����������Ʊ���
ij��ȤС����FeCl2��NH4HCO3�Ʊ�FeCO3��װ��ʾ��ͼ��ͼ��
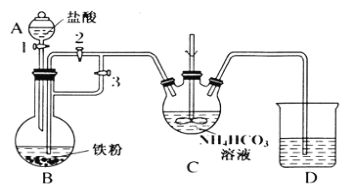
�ش��������⣺
(1)Cװ�����漰����Ҫ��Ӧ�����ӷ���ʽ_________��
(2)��D�������崿�������ɵ�FeCl2��Һ��NH4HCO3��Һ���ʱ�IJ�����_____��
(3)���Ƶõ�FeCO3���뵽����������Һ�У��ټ����������ۣ�75���½��跴Ӧ�������������۵�������_______��
(4)��Ӧ������������ˣ���ȥ�������۵ķ�����_________��
(5)��������Һ�л��������������ķ����ǣ�________����ȴ�ᾧ�����ˣ� �������Ҵ�ϴ�ӣ����
��.�����������崿�ȵIJ�����
(6)����ȤС����KMnO4�ζ����ⶨ��Ʒ�������������������Ʒ�������������������������ֲ�Ʒ�������������Ǵ���100%����ԭ�������___��
(7)������������ȤС�������(Ce)�����ⶨ��Ʒ��Fe2+�ĺ������ζ���Ӧ���£�Ce4++Fe2+=Ce3++Fe3+��ȡ1.440g��Ʒ���100mL��Һ��ÿ��ȡ20.00mL�����б�Ҫ��������0.0500molL-1Ce(SO4)2����Һ�ζ����յ㣬��¼���������
�ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
1 | 0.20 | 19.95 |
2 | 0.10 | 21.65 |
3 | 0.95 | 20.60 |
���Ʒ��������������������Ϊ________%��(С�������һλ����)
(8)�����ʵ��֤���㹺������������������к�Fe2+��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Է�������Ϊ40����״��������A���ܷ�������ת����
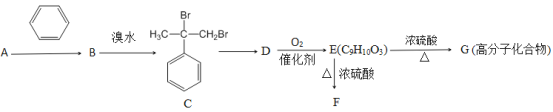
��֪�����ǻ������ӵ�̼ԭ��������ԭ��ʱ�����Է�������������E����NaHCO3��Һ��Ӧ�������壻��FΪ������
(1)A�ķ���ʽΪ_____________��Aת��ΪB�ķ�Ӧ����Ϊ______________��Cת��ΪD�ķ�Ӧ����Ϊ_________________��
(2)B�������еĹ����ŵ�����Ϊ_____________��B�й�ƽ���ԭ�������________����
(3)д��Dת��ΪE�Ļ�ѧ����ʽ��__________________
(4)��E��Ϊͬ���칹��ķ����廯����K����֪����K��E������ͬ�Ĺ����ţ���K������FeCl3��Һ������ɫ��Ӧ����K�ж���ȡ���������������������K����_________��(����ĸ����)
a.3�� b.6�� c. 9�� d.12��
(5)F�Ľṹ��ʽΪ_________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com