
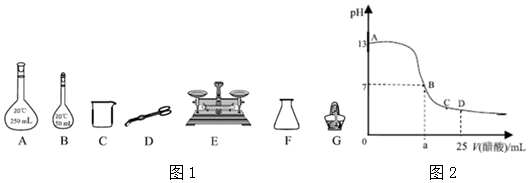
 ŹµŃéŹŅijÅØĮņĖįµÄŹŌ¼ĮĘæÉĻµÄ±źĒ©ČēÓŅĶ¼ĖłŹ¾£¬øł¾Ż±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ
ŹµŃéŹŅijÅØĮņĖįµÄŹŌ¼ĮĘæÉĻµÄ±źĒ©ČēÓŅĶ¼ĖłŹ¾£¬øł¾Ż±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ·ÖĪö £Ø1£©ŅĄ¾ŻC=$\frac{1000¦Ń¦Ų}{M}$¼ĘĖćÅØĮņĖįµÄĪļÖŹµÄĮæÅØ¶Č£»
£Ø2£©ŅĄ¾ŻÅäÖĘČÜŅŗĢå»żŃ”ŌńČŻĮæĘæ¹ęøń£¬ŅĄ¾ŻČÜŅŗĻ”ŹĶ¹ż³ĢÖŠČÜÖŹµÄĪļÖŹµÄĮæ²»±ä¼ĘĖćŠčŅŖÅØĮņĖįµÄĢå»ż£»
£Ø3£©A£®ŅĄ¾Żm=CVM¼ĘĖćŠčŅŖČÜÖŹµÄÖŹĮ棻
B£®³ĘĮæøÆŹ“ŠŌŅ©Ę·£¬Ó¦·ÅŌŚŠ”ÉÕ±»ņÕß³ĘĮæĘæ ÖŠ£»
C£®ŅĄ¾ŻĮæĶ²¾«Č·¶ČÅŠ¶Ļ£»
D£®ŅĄ¾ŻÅäÖĘČÜŅŗĢå»żŗĶ³£ÓĆČŻĮæĘæ¹ęøńÅŠ¶Ļ£»
E£®ŅĄ¾ŻČÜŅŗĻ”ŹĶ¹ęĀɽā“š£»
£Ø4£©·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©ÅØĮņĖįĪļÖŹµÄĮæÅضČC=$\frac{1000”Į1.84”Į98%}{98}$=18.4mol/L£»
¹Ź“š°øĪŖ£ŗ18.4£»
£Ø2£©ÅäÖĘ920mLĪļÖŹµÄĮæÅضČĪŖ0.30mol•L-1µÄĻ”ĮņĖį£¬Ć»ÓŠ920mLČŻĮæĘæ£¬Ó¦Ń”Ōń1000mLČŻĮæĘ棬ÉčŠčŅŖÅØĮņĖįĢå»żĪŖV£¬ŌņŅĄ¾ŻČÜŅŗĻ”ŹĶ¹ż³ĢÖŠČÜÖŹµÄĪļÖŹµÄĮæ²»±äµĆ£ŗ18.4mol/L”ĮV=0.30mol•L-1”Į1000mL£¬½āµĆV=16.3mL£»
¹Ź“š°øĪŖ£ŗ1000£» 16.3£»
£Ø3£©A£®ÅäÖĘ0.1mol/L CuSO4ČÜŅŗ100mL£¬Šč³ĘĮæCuSO4•5H2O ÖŹĮæm=0.1mol/L”Į0.1L”Į250g/mol=2.5g£¬¹ŹA“ķĪó£»
B£®ĒāŃõ»ÆÄĘ¾ßÓŠøÆŹ“ŠŌ£¬Ó¦·ÅŌŚŠ”ÉÕ±»ņÕß³ĘĮæĘæÖŠ½ųŠŠ£¬¹ŹB“ķĪó£»
C£®ĮæĶ²¾«Č·¶ČĪŖ0.1mL£¬ĖłŅŌ¶ĮŹżŹ±Š”ŹżµćŗóÓ¦±£Įō1Ī»Š”Źż£¬¹ŹC“ķĪó£»
D£®ŠčŅŖ235mL 0.9mol/L NaClČÜŅŗ£¬ŹµŃéŹŅƻӊ235mLČŻĮæĘæ£¬Ó¦Ń”Ōń250mLČŻĮæĘ棬¹ŹDÕżČ·£»
E£®½«ÅäÖĘŗĆŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ£¬×¢ČėøÕÓĆĖ®Ļ“¾»µÄŹŌ¼ĮĘæÖŠ£¬µ¼ÖĀČÜŅŗ±»Ļ”ŹĶ£¬ČÜŅŗÅضČĘ«Š”£¬¹ŹE“ķĪó£»
¹ŹŃ”£ŗD£»
£Ø4£©A£®ČŻĮæĘæÓĆÕōĮóĖ®Ļ“¾»ŗó£¬Ī“µČÄŚ±ŚøÉŌļ±ćÓĆĄ“ÅäÖĘ£¬¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»ż¶¼²»»į²śÉśÓ°Ļģ£¬ČÜŅŗÅØ¶Č²»±ä£¬¹ŹA²»Ń”£»
B£®ÉÕ¼īŌŚÉÕ±ĄļøÕŗĆĶźČ«Čܽā£¬Į¢¼“°ŃČÜŅŗ×ŖŅʵ½ČŻĮæĘæÖŠ£¬ĄäČ“ŗóČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹB²»Ń”£»
C£®¶ØČŻŹ±£¬ŃöŹÓæĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹCŃ”£»
D£®Ņ”ŌČ¾²ÖĆŗ󣬷¢ĻÖŅŗĆęĪ“µ½æĢ¶ČĻߣ¬¼ĢŠų²¹¼ÓĖ®ÖĮæĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹDŃ”£»
E£®ŌŚČܽā¹ż³ĢÖŠÓŠÉŁĮæŅŗĢ彦³öÉÕ±Ķā£¬µ¼ÖĀČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹEŃ”£»
¹ŹŃ”£ŗCDE£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ£¬Ć÷Č·ÅäÖĘŌĄķ¼°²Ł×÷²½ÖčŹĒ½āĢā¹Ų¼ü£¬×¢ŅāĪó²ī·ÖĪöµÄ·½·ØŗĶ¼¼ĒÉ£¬ĢāÄæÄŃ¶Č²»“ó£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé²Ł×÷ | ĻÖĻó | ½įĀŪ |
| A | ĻņŹ¢ÓŠ1mLÅØĮņĖįµÄŹŌ¹ÜÖŠ¼ÓČė5mL 0.1mol•L-1 µÄ K2Cr2O7ČÜŅŗ | ČÜŅŗ³ČÉ«±äÉī | “óÉś³ÉĪļÅØ¶Č£¬Ę½ŗāCr2O72-£Ø³ČÉ«£©+H2O?2CrO42- £Ø»ĘÉ«£©+2H+ÄęĻņŅĘ ¶Æ |
| B | ĻņMg£ØOH£©2Šü×ĒŅŗÖŠ¼ÓČėÉŁĮæ ĀČ»Æļ§¾§Ģå | ³ĮµķČܽā£¬ČÜŅŗ±ä³ĪĒå | ĖµĆ÷·“Ó¦Mg£ØOH£©2+2NH4+?Mg2++2NH3•H2O¾ßÓŠæÉÄęŠŌ |
| C | °×ĢśĘ¤£Ø¶ĘŠæĢś£©³öĻÖ¹ĪŗŪŗó½žÅŻ ŌŚ±„ŗĶŹ³ŃĪĖ®ÖŠ£¬Ņ»¶ĪŹ±¼äŗóµĪČė¼øµĪK3[Fe£ØCN£©6]ČÜŅŗ | ĪŽĆ÷ĻŌĻÖĻó | øĆ¹ż³ĢĪ“·¢ÉśŌµē³Ų·“Ó¦ |
| D | ĻņĆŗĀÆÖŠ×ĘČȵÄĆŗĢæÉĻČ÷ÉŁĮæĖ® | ²śÉśµĄ¶É«»š Ńę£¬ĆŗĢæČ¼ÉÕøüĶś | ¼ÓÉŁĮæĖ®ŗó£¬æÉŹ¹ĆŗĢæČ¼Éշųöøü¶ąµÄČČĮæ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÖŹ×ÓŹżĪŖ53£¬ÖŠ×ÓŹżĪŖ78µÄµāŌ×Ó£ŗ53131I | |
| B£® | CH4·Ö×ӵıȥżÄ£ŠĶ£ŗ | |
| C£® | Na+ µÄ½į¹¹Ź¾ŅāĶ¼£ŗ | |
| D£® | NaOHµÄµēĄė·½³ĢŹ½£ŗNaOH?Na++OH- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŃĻ½ūĀ¶Ģģ·ŁÉÕÅ©×÷Īļ½ÕøŃ | |
| B£® | ¼ÓĒæÖ²Ź÷ŌģĮÖ£¬Ōö“óĀĢ»ÆĆ껿 | |
| C£® | “óĮ¦ĶĘŠŠĘū³µŹ¹ÓĆĢģČ»Ęų»ņĒāĘųĪŖČ¼ĮĻ | |
| D£® | Éś²śÉś»īÖŠ¼Ó“óĆŗĢæŗĶŹÆÓĶµČČ¼ĮĻµÄŹ¹ÓĆĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĘšŹ¼ĪĀ¶Č/”ę | ÖÕÖ¹ĪĀ¶Č/”ę | ĪĀ¶Č²ī/”ę | |||
| H2SO4ČÜŅŗ | NaOHČÜŅŗ | Ę½¾łÖµ | |||
| 1 | 25.0 | 24.5 | 24.75 | 29.3 | 4.55 |
| 2 | 24.5 | 24.2 | 24.35 | 28.3 | 3.95 |
| 3 | 25.0 | 24.5 | 24.75 | 28.7 | 3.95 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µČÖŹĮæµÄĮņÕōĘųŗĶĮņ¹ĢĢå·Ö±šĶźČ«Č¼ÉÕ£¬ŗóÕ߷ųöµÄČČĮæ¶ą | |
| B£® | ŠčŅŖ¼ÓČČ²ÅÄÜ·¢ÉśµÄ·“Ó¦Ņ»¶ØŹĒĪüČČ·“Ó¦ | |
| C£® | ŌŚ101 kPaŹ±£¬2 g H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®£¬·Å³ö285.8 kJČČĮ棬ĒāĘųČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ2H2£Øg£©+O2£Øg£©ØT2H2O£Øl£©”÷H=+285.8 kJ/mol | |
| D£® | Ļ”ČÜŅŗÖŠ£ŗH+£Øaq£©+OH-£Øaq£©ØTH2O£Øl£©”÷H=-57.3 kJ/mol£¬ÅØH2SO4Óėŗ¬1 mol NaOHµÄČÜŅŗ»ģŗĻ£¬·Å³öµÄČČĮæ“óÓŚ57.3 kJ/mol |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com