
(1)���һ֧�Թ��е����̪��Һ����Һ��졣
(2)��ڶ�֧�Թ��м�������Ba(NO3)2��Һ�����ɰ�ɫ���������ˣ�������Һ�������֧�Թܣ��ڳ����м���ϡ���ᣬ��������ʧ������ɫ��ζ���������������ͨ�����ʯ��ˮ��ʯ��ˮ����ǡ�
(3)�����֧�Թ��м�������AgNO3��Һ������
ͨ������ʵ����ж���Һ�к��е�������___________________________���϶������е�������________________________����ȷ���Ƿ��е�������___________________��д��ʵ��(2)���йط�Ӧ�����ӷ���ʽ��_________________________��
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������ƺ��ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��֪ij��̬��ʯȼ��X��ֻ����̼��������Ԫ�أ�Ϊ̽����������̼��������Ԫ�ص������ȣ�ijͬѧ�����ȼ�շ�������ʵ�鷽����ͨ������װ��C��D�����ؼ������̼��������Ԫ�ص������ȡ�ʵ��װ����ͼ��ʾ(��֪CuO������Ϊ̼�⻯����ȼ�յĴ���)��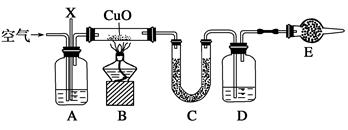
(1)��Aװ������ʢ�ŵ�ҩƷ��ŨNaOH ��Һ��д��װ��A��һ�����ã�_
(2)Cװ������ʢ�ŵ�ҩƷ�ǣ�
(3)Dװ������ʢ�ŵ�ҩƷ�ǣ�
(4)Eװ������ʢ�ŵ�ҩƷ�ǣ�
(5)����װ������һ������ (������
�ƾ��ƺͼ��ȷ������ܴ��ڵĴ���
������������ҩƷ����ָ��������ҩ
Ʒ���ƺ�λ��)�������������
(6) ��ʵ��װ�þ��������������¶���ʵ�飺ȷ��ȡ7.2 g��Ʒ��ֻ��C��H��O����Ԫ���е����ֻ����֣��������ȼ�պ�U�ι�C����������10.8 g�����ƿD��������22 g������л�������ʽΪ
��7������ȷ�������ʽ������ͬ���칹���зе�������ʵ�����__ �� (ϰ��������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��֪ij��̬��ʯȼ��X��ֻ����̼��������Ԫ�أ�Ϊ̽����������̼��������Ԫ�ص������ȣ�ijͬѧ�����ȼ�շ�������ʵ�鷽����ͨ������װ��C��D�����ؼ������̼��������Ԫ�ص������ȡ�ʵ��װ����ͼ��ʾ(��֪CuO������Ϊ̼�⻯����ȼ�յĴ���)��

(1)��Aװ������ʢ�ŵ�ҩƷ��ŨNaOH ��Һ��д��װ��A��һ�����ã�_
(2)Cװ������ʢ�ŵ�ҩƷ�ǣ�
(3)Dװ������ʢ�ŵ�ҩƷ�ǣ�
(4)Eװ������ʢ�ŵ�ҩƷ�ǣ�
(5)����װ������һ������ (������
�ƾ��ƺͼ��ȷ������ܴ��ڵĴ���
������������ҩƷ����ָ��������ҩ
Ʒ���ƺ�λ��)�������������
(6) ��ʵ��װ�þ��������������¶���ʵ�飺 ȷ��ȡ7.2 g��Ʒ��ֻ��C��H��O����Ԫ���е����ֻ����֣��������ȼ�պ�U�ι�C����������10.8 g�����ƿD��������22 g������л�������ʽΪ
��7������ȷ�������ʽ������ͬ���칹���зе�������ʵ�����__ �� (ϰ��������)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com