
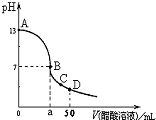
 ��25mL0.1mol∙L-1 NaOH��Һ����μ���0.1mol∙L-1 CH3COOH��Һ����������ͼ��ʾ���й�����Ũ�ȹ�ϵ��ȷ����
��25mL0.1mol∙L-1 NaOH��Һ����μ���0.1mol∙L-1 CH3COOH��Һ����������ͼ��ʾ���й�����Ũ�ȹ�ϵ��ȷ����
A����A��B����һ�㣬��Һ��һ������c(Na+)+c(H+)=c(CH3COO��)+(OH��)
B����B�㣬a��25������c(Na+)=c(CH3COO��)=c(OH��)=c(H+)
C����C�㣺c(CH3COO��)��c(Na+)��c(H+)��c(OH��)
D����D�㣺c(CH3COO��)+c(CH3COOH)=c(Na+)
AC
�������:��ѡB��D��ѡB��ΪNaOH��Һ�м���CH3COOH��Һ���������൱ʱǡ������CH3COO Na������ʱ��ҺΪ���ԣ���ֻ�д��������a��25�����ڵ���غ�c(Na+) +c(H+)=c(CH3COO��)+c(OH��)���ִ�ʱ��ҺΪ���ԣ����c(OH��)=c(H+)��c(Na+)=c(CH3COO��)��ѡD��Ϊ��D�㿴��Ϊ��Һ�����Ϊ50ml,�����ӵ����͵�����Һ�д�����ڵ�������ʽ��
�γɴ���:��ѡBû������������Һ��c(OH��)=c(H+)=10-7 mol/LԶС��c Na+����ѡD��ͼ�ĺ�����û�����⡣����ԭ���Ƕ���Һ������Ũ�ȴ�С�Ƚϵ�ʵ�ʲ��ܺܺ����ա�
����ָ��:A�������ݵ���غ�˹�ϵʽ��ȷ��B����NaOH��Һ�м���CH3COOH��Һ���������൱ʱǡ������CH3COONa������ʱ��ҺΪ���ԣ���ֻ�д��������a��25�����ڵ���غ�c(Na+) +c(H+)=c(CH3COO��)+c(OH��)���ִ�ʱ��ҺΪ���ԣ����c(OH��)=c(H+)��c(Na+)=c(CH3COO��) ��������Һ��c(OH��)=c(H+)=10-7 mol/LԶС��c Na+��B�����C����C����ҺΪ�����ƺʹ���Ļ����Һ����ֱC��ȷ��D��ӦΪc(CH3COO��)+c(CH3COOH)=2c(Na+)


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
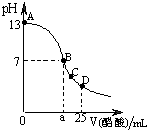 ��25mL0.1mol/LNaOH��Һ����μ���0.2mol/L������Һ��������ͼ��ʾ���й�����Ũ�ȹ�ϵ�Ƚϲ���ȷ�ģ�������
��25mL0.1mol/LNaOH��Һ����μ���0.2mol/L������Һ��������ͼ��ʾ���й�����Ũ�ȹ�ϵ�Ƚϲ���ȷ�ģ�������| A����A��B����һ�㣬��Һ��һ������c��Na+����c��CH3COO-����c��OH-����c��H+�� | B����B�㣬a��12.5������c��Na+��=c��CH3COO-��=c��OH-��=c��H+�� | C����C�㣺c��CH3COO-����c��Na+����c��H+����c��OH-�� | D����D�㣺c��CH3COO-��+c��CH3COOH��=2c��Na+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��25mL0.1mol��LNaOH��Һ����μ���0.2mol��L������Һ����������ͼ��ʾ���й�����Ũ�ȹ�ϵ�Ƚ���ȷ�� ( )
��25mL0.1mol��LNaOH��Һ����μ���0.2mol��L������Һ����������ͼ��ʾ���й�����Ũ�ȹ�ϵ�Ƚ���ȷ�� ( )
A����A��B����һ�㣬��Һ��һ������c(Na+)>c(CH3COO-)>c(OH-)>c(H+)
B����B�㣬a>12.5������c(Na+) = c(CH3COO-) = c(OH-) = c(H+)
C����C�㣺c(CH3COO-)> c(Na+)>c(H+)>c(OH-)
D����D�㣺c(CH3COO-) + c(CH3COOH) = 2c(Na+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25��ʱ����25mL0.1mol/L������������Һ�У���μ���0.2mol/LCH3COOH����Һ��pH�ı仯������ͼ��ʾ�����з����Ľ�����ȷ����
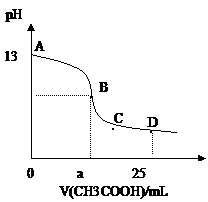 A.B������a==12.5������c(Na+)==c(CH3COO��)
A.B������a==12.5������c(Na+)==c(CH3COO��)
B.C��ʱ��c(CH3COO��)��c(Na+)��c(H+)= c(OH��)
C.D��ʱ��c(CH3COO��)+ c(CH3COOH)==2c(Na+)
D.��������A��B���κ�һ�㣬��Һ�ж��У�
c(Na+)��c(CH3COO��)��c(OH��) ��c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25��ʱ����25mL0.1mol/L������������Һ�У���μ���0.2mol/LCH3COOH����Һ��pH�ı仯������ͼ��ʾ�����з����Ľ�����ȷ����
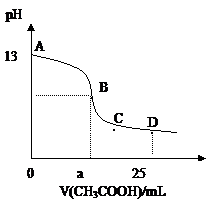 A.B������a==12.5������c(Na+)==c(CH3COO��)
A.B������a==12.5������c(Na+)==c(CH3COO��)
B.C��ʱ��c(CH3COO��)��c(Na+)��c(H+)= c(OH��)
C.D��ʱ��c(CH3COO��)+ c(CH3COOH)==2c(Na+)
D.��������A��B���κ�һ�㣬��Һ�ж��У�
c(Na+)��c(CH3COO��)��c(OH��) ��c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ĸ߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ѡ����
��25mL0.1mol∙L-1 NaOH��Һ����μ���0.1mol∙L-1 CH3COOH��Һ��������ͼ��ʾ���й�����Ũ�ȹ�ϵ��ȷ����
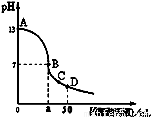
A����D�㣺c(CH3COO��)+c(CH3COOH)=c(Na+)
B����B�㣬a��25������c(Na+)=c(CH3COO��)=c(OH��)=c(H+)
C����C�㣺c(Na+)��c(CH3COO��) ��c(OH��)��c(H+)
D����A��B����һ�㣬��Һ��һ������c(Na+)+c(H+)=c(CH3COO��)+(OH��)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com