
(2009����������)W��X��Y��Z�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������W��Y���������ǵ����������Ҫ���ʣ�X�Ļ�̬ԭ�Ӻ�����7��ԭ�ӹ������˵��ӣ�Z���γɺ�ɫ(��ש��ɫ)��Z2O�ͺ�ɫ��ZO���������
(1)Wλ��Ԫ�����ڱ���_____���ڵ�______�塣W����̬�⻯���ȶ��Ա�H2O(g)_____(�ǿ��������)��
(2)Y��̬ԭ�Ӻ�������Ų�ʽ��________��Y��һ�����ܱ�X��___ (���С)
(3)Y������������Ӧˮ�����Ũ��Һ��Z�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(4)��֪�������ݣ�Fe(s)��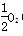 (g)===FeO(s)����H����272.0 kJ��mol��1
(g)===FeO(s)����H����272.0 kJ��mol��1
2X(s)��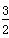 O2(g)===X2O3(s)����H����1 675.7 kJ��mol��1
O2(g)===X2O3(s)����H����1 675.7 kJ��mol��1
X�ĵ��ʺ�FeO��Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
��11�֣�(1)������A�� ���� (2)1s22s22p63s23p4����
(3)Cu��2H2SO4(Ũ) CuSO4��SO2����2H2O��
CuSO4��SO2����2H2O��
(4)3FeO(s)��2Al(s)===Al2O3(s)��3Fe(s)����H����859.7 kJ��mol��1
��������
���������W��Y���������ǵ����������Ҫ���ʣ���W��NԪ�أ�Y��SԪ�ء�X�Ļ�̬ԭ�Ӻ�����7��ԭ�ӹ������˵��ӣ���ԭ������С��S�ģ�����X��AlԪ�ء�Z���γɺ�ɫ(��ש��ɫ)��Z2O�ͺ�ɫ��ZO�����������Z��Cu��
��1����Ԫ��λ�ڵڶ����ڵڢ�A�塣��Ԫ�صķǽ�����������Ԫ�صģ����������ȶ���С��H2O�ġ�
��2�����ݹ���ԭ����֪��SԪ�ػ�̬ԭ�ӵĺ�������Ų�ʽ��1s22s22p63s23p4���ǽ�����Խǿ����һ������Խ����SԪ�صĵ�һ�����ܴ���AlԪ�صĵ�һ�����ܡ�
(3)Y������������Ӧˮ�����Ũ��Һ��Ũ���ᣬ������Cu�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ��Cu��2H2SO4(Ũ) CuSO4��SO2����2H2O��
CuSO4��SO2����2H2O��
��4�����ݸ�˹���ɿ�֪���١�3���ڼ��õ�3FeO(s)��2Al(s)===Al2O3(s)��3Fe(s)�����Ը÷�Ӧ�ķ�Ӧ�Ȧ�H����272.0 kJ��mol��1��3��1 675.7 kJ��mol��1����859.7 kJ��mol��1��
���㣺����Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ���Լ�����ʽ����д�ͷ�Ӧ�ȵļ���
�������������е��Ѷȵ����⣬Ҳ�Ǹ��еij�����������͡������ۺ���ǿ���������У����ض�ѧ�������������ͽ��ⷽ���뼼�ɵ�ָ����ѵ����������Ҫ���ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣������̩��һ�и߶��µ�һ�νο��Ի�ѧ�Ծ����������� ���ͣ������
(2009����������)W��X��Y��Z�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������W��Y���������ǵ����������Ҫ���ʣ�X�Ļ�̬ԭ�Ӻ�����7��ԭ�ӹ������˵��ӣ�Z���γɺ�ɫ(��ש��ɫ)��Z2O�ͺ�ɫ��ZO���������
(1)Wλ��Ԫ�����ڱ���_____���ڵ�______�塣W����̬�⻯���ȶ��Ա�H2O(g)_____(�ǿ��������)��
(2)Y��̬ԭ�Ӻ�������Ų�ʽ��________��Y��һ�����ܱ�X��___ (���С)
(3)Y������������Ӧˮ�����Ũ��Һ��Z�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(4)��֪�������ݣ�Fe(s)�� (g)===FeO(s)����H����272.0 kJ��mol��1
(g)===FeO(s)����H����272.0 kJ��mol��1
2X(s)��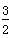 O2(g)===X2O3(s)����H����1 675.7 kJ��mol��1
O2(g)===X2O3(s)����H����1 675.7 kJ��mol��1
X�ĵ��ʺ�FeO��Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com