
���� һ���¶��£������Ϊ2L���ܱ������м���1mol N2��3mol H2����2min��ﵽƽ�⣬ƽ��ʱ���NH3��Ũ��Ϊ0.5mol/L����
N2��g��+3H2��g��?2NH3��g��
��ʼ 0.5 1.5 0
ת�� 0.25 0.75 0.5
��1�����v=$\frac{��c}{��t}$���㣻
��2���ϳɰ���ӦΪ���淴Ӧ��������ȫת����
��3�����ƽ����������ȡ��������������������ж�ƽ��״̬��
��4����N2��g��+2O2��g��=2NO2��g����H1=+67.7kJ/mol
��N2H4��g��+O2��g��=N2��g��+2H2O��g����H2=-534.0kJ/mol
��ϸ�˹���ɿ�֪�ڡ�3-�ٵõ�2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����
��� �⣺��1��2min ��H2�ķ�Ӧ����v��H2��=$\frac{0.75mol/L}{2min}$=0.375 mol/��L•min�����ʴ�Ϊ��0.375 mol/��L•min����
��2����ַ�Ӧ���ﵽƽ��ʱ���ų�������С��92.3kJ�����淴Ӧ����Ϊ��̬ƽ�⣬ʼ�մﲻ�����ų�������
�ʴ�Ϊ��С�ڣ� ���淴Ӧ����Ϊ��̬ƽ�⣬ʼ�մﲻ�����ų�������
��3��A����λʱ���ڣ��Ͽ�1mol N��N��ͬʱ�Ͽ�3mol H-H��ֻ��������Ӧ���ʹ�ϵ�������ж�ƽ�⣬��A��ѡ��
B����λʱ���ڣ��γ�1mol N��N��ͬʱ�γ�3mol N-H��ֻ�������淴Ӧ���ʹ�ϵ�������ж�ƽ�⣬��B��ѡ��
C����λʱ���ڣ��Ͽ�1mol N��N��ͬʱ�Ͽ�6mol N-H����֪���淴Ӧ������ȣ�Ϊƽ��״̬����Cѡ��
D����λʱ���ڣ��γ�1mol N��N��ͬʱ�Ͽ�3mol H-H����֪���淴Ӧ������ȣ�Ϊƽ��״̬����Dѡ��
�ʴ�Ϊ��C��D��
��4����N2��g��+2O2��g��=2NO2��g����H1=+67.7kJ/mol
��N2H4��g��+O2��g��=N2��g��+2H2O��g����H2=-534.0kJ/mol
��ϸ�˹���ɿ�֪�ڡ�3-�ٵõ�2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=��-534.0kJ/mol����2-��+67.7kJ/mol��=-947.6 kJ•mol-1�����Ȼ�ѧ����ʽΪ2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=-947.6 kJ•mol-1��
�ʴ�Ϊ��2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=-947.6 kJ•mol-1��
���� ���⿼�黯ѧƽ����㣬Ϊ��Ƶ���㣬�������ʼ��㡢ƽ���ж�����˹����Ӧ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע���˹�������ʱ�����е�Ӧ�ã���Ŀ�ѶȲ���


 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �٢ڢ� | B�� | �ܢݢ� | C�� | �ڢܢ� | D�� | �ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �Ըߴ�H2Ϊȼ�ϵ����ӽ���Ĥȼ�ϵ�ؾ�������Ч�ʸߡ�����Ⱦ���ŵ㣬��ȼ����������CO���������̵���������Լ״�Ϊԭ����ȡ�ߴ�H2����Ҫ�о�����
�Ըߴ�H2Ϊȼ�ϵ����ӽ���Ĥȼ�ϵ�ؾ�������Ч�ʸߡ�����Ⱦ���ŵ㣬��ȼ����������CO���������̵���������Լ״�Ϊԭ����ȡ�ߴ�H2����Ҫ�о������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
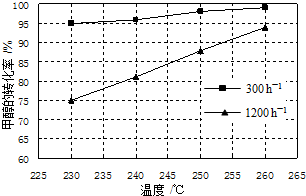
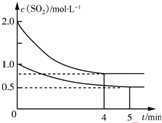 ��ס��������ݻ���Ϊ1L�ĺ��º��ݵ��ܱ������У��ֱ����һ������SO2��O2�����У��׳���2mol SO2��1mol O2���ҳ���1mol SO2��0.5mol O2����������Ӧ��2SO2��g��+O2��g��?2SO3��g����H=-197.74kJ•mol-1��һ��ʱ���ﵽƽ�⣬�����������c��SO2����mol•L-1����ʱ��t��min���ı仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
��ס��������ݻ���Ϊ1L�ĺ��º��ݵ��ܱ������У��ֱ����һ������SO2��O2�����У��׳���2mol SO2��1mol O2���ҳ���1mol SO2��0.5mol O2����������Ӧ��2SO2��g��+O2��g��?2SO3��g����H=-197.74kJ•mol-1��һ��ʱ���ﵽƽ�⣬�����������c��SO2����mol•L-1����ʱ��t��min���ı仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �ų�������Q��Q���ף���2Q���ң� | |
| B�� | ��ϵ��ѹǿp��p���ף���2p���ң� | |
| C�� | ����ǰ5 min�ڵķ�Ӧ����v��O2��=0.05mol•L-1•min-1 | |
| D�� | ���������������䣬����ʼʱ�����г���0.4 mol SO2��0.2 mol O2��0.4 mol SO3�����ʱv��������v���棩 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
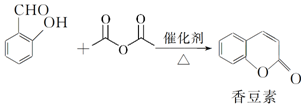

 ��
�� ��
��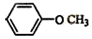 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
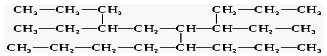
| A�� | 8 | B�� | 9 | C�� | 11 | D�� | 12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

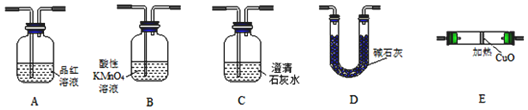
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.50 | 25.45 |
| �ڶ��� | 20.00 | 4.00 | 29.05 |
| ������ | 20.00 | 3.00 | 30.00 |
| ���Ĵ� | 20.00 | 2.00 | 27.00 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com