
����Ŀ���������£�����������Һ��
��0.1mol/L NH4Cl ��0.1mol/L CH3COONH4 �� 0.1mol/L NH4HSO4
��0.1mol/L NH3H2O��0.1mol/L NH4Cl���Һ ��0.1mol/L NH3H2O
�����Ҫ����д���пհף�
��1����Һ�ٳ�_________�ԣ���ᡱ��������С�������ԭ����_____________�������ӷ���ʽ��ʾ����
��2��������������Һ�У�pH��С����_____��c��NH4+����С����____������ţ���
��3������Һ���У�_____________���ӵ�Ũ��Ϊ0.1mol/L��NH3H2O��________���ӵ����ʵ���Ũ��֮��Ϊ0.2 mol/L��
��4�������£������Һ�ڵ�pH=7����CH3COO����NH4+Ũ�ȵĴ�С��ϵ�ǣ�c��CH3COO����_____________c��NH4+�������������������������
���𰸡��� NH4++H2O![]() H++NH3H2O �� �� Cl- NH4+ =
H++NH3H2O �� �� Cl- NH4+ =
��������
����ˮ��һ�������ģ�����Ӱ��������ˮ�����ӵ����أ����������ʵĵ��룬�ж���Һ��������Լ��������Ũ�ȵĴ�С��HSO4-�������H+����NH4+��ˮ�⣻�����ʵ���Ũ�ȵ�NH3H2O��0.1mol/L NH4Cl���Һ��NH3H2O�ĵ������NH4+ˮ�⣬��Һ�ʼ��ԣ�NH4+��CH3COO-��ˮ�⣬����ˮ����ٽ��̶���ͬ��
��1��NH4Cl��ǿ�������Σ�NH4+ˮ�������ԣ�NH4+ˮ�ⷴӦ�����ӷ���ʽNH4++H2O![]() H++NH3H2O��
H++NH3H2O��
��2������Һ��������Ũ��Խ����ҺpHԽС����0.1mol/L NH4Cl��NH4+ˮ�������ԣ�Cl-��ˮ�⣻��CH3COONH4�����������Σ�0.1mol/L CH3COONH4��NH4+��CH3COO-��ˮ�⣬����ˮ����ٽ��̶���ͬ����Һ�����ԣ��� 0.1mol/L NH4HSO4��HSO4- = H++SO42-��HSO4-�������H+����NH4+��ˮ�⣻��0.1mol/L NH3H2O��0.1mol/L NH4Cl���Һ��NH3H2O�ĵ������NH4+ˮ�⣬��Һ�ʼ��ԣ���Һ��NH4+ˮ���Ũ�ȴ���0.1mol/L����0.1mol/L NH3H2O��NH3H2O��������ʣ�NH3H2O![]() NH4++OH-�� NH3H2O����������NH4+��������������֪��ҺpH��С���Ǣ� 0.1mol/L NH4HSO4��c(NH4+)��С���Ǣ�0.1mol/L NH3H2O��
NH4++OH-�� NH3H2O����������NH4+��������������֪��ҺpH��С���Ǣ� 0.1mol/L NH4HSO4��c(NH4+)��С���Ǣ�0.1mol/L NH3H2O��
��3����0.1mol/L NH3H2O��0.1mol/L NH4Cl���Һ�������ӵ����ʵ������䡢Ũ�Ȳ��䣬�����ӵ�Ũ��Ϊ0.1mol/L��NH3H2O�ĵ������NH4+ˮ�⣬��Һ�ʼ��ԣ��������غ��֪��c(NH3H2O)+c(NH4+)=0.2 mol/L��
��4�������£������Һ�ڵ�pH=7��NH4+��CH3COO-��ˮ�⣬����ˮ����ٽ��̶���ͬ��c(CH3COO-)=c(NH4+)��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C��N��P��S��Fe��Cu������������������ϢϢ��صĻ�ѧԪ�أ�������ѧ֪ʶ�ش��������⣺
��1����̬Cuԭ�Ӻ�������Ų�ʽΪ___��������µ��ȶ���CuO___Cu2O����������������������
��2�����о�Cu��ij�ֻ������ܴ�����CO(NH2)2������C��N���ӻ���ʽ�ֱ�Ϊ___��___��
��3��Si��P��SԪ�صĵ�һ�������ɴ�С��˳����___��
��4��OF2�Ŀռ乹����___�����Ӽ��ԣ�H2O___OF2����������������������������___��
��5��[Fe(CN)6]3-����λ���������ĸ���֮��Ϊ___��
��6�����ǻ�����ȩ�ķе���ڶ��ǻ�����ȩ�ķе㣬ԭ����___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ������![]() ��Ũ����Cl-��Ũ�ȱ�Ϊ1:1����Һ������NH4Cl��Һ�м���
��Ũ����Cl-��Ũ�ȱ�Ϊ1:1����Һ������NH4Cl��Һ�м���
��������HCl ��������NaCl �������İ�ˮ ��������NaOH
A.�٢�B.��C.�ۢ�D.��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�÷�Ӧ4NH3+5O2=4NO+6H2O�У�����Ӧ���ʷֱ���v��NH3����v��O2����v��NO����v��H2O����ʾ������ȷ�Ĺ�ϵ��
A.![]() v��NH3��=v��O2��B.
v��NH3��=v��O2��B.![]() v��O2��=v��H2O��
v��O2��=v��H2O��
C.![]() v��NH3��=v��H2O��D.
v��NH3��=v��H2O��D.![]() v��O2��=v��NO��
v��O2��=v��NO��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����г�����Ũ�Ⱦ�Ϊ![]() ������5����Һ��
������5����Һ��![]() ��Һ
��Һ![]() ��Һ
��Һ![]() ��Һ
��Һ![]() ��Һ
��Һ![]() ��Һ
��Һ
![]() ��5����ҺpH�ɴ�С��˳����________________��������ˮ�����
��5����ҺpH�ɴ�С��˳����________________��������ˮ�����![]() Ũ����С����______��
Ũ����С����______��![]() �����
�����![]()
![]() �и�����Ũ���ɴ�С��˳����________________________________________________________��
�и�����Ũ���ɴ�С��˳����________________________________________________________��![]() ��ˮ��ƽ�ⳣ��
��ˮ��ƽ�ⳣ��![]() _________________��
_________________��![]() ��֪̼��ĵ��볣��
��֪̼��ĵ��볣��![]() ��
��![]()
![]() ��
��![]() ��ͨ��������������ʱ
��ͨ��������������ʱ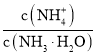 ��ֵ__________
��ֵ__________![]() ����������������С������������
����������������������������![]() ��
��
![]() ����
����![]() ��
��![]() ��Ϻ���Һǡ�ó����ԣ�����ǰ
��Ϻ���Һǡ�ó����ԣ�����ǰ![]() �����______
�����______![]() �����
�����![]() ����������������������������
����������������������������![]() ��
��
![]() ��
��![]() ����Һ�ֱ����ɲ����գ����տɵ�ԭ���ʵ���____________
����Һ�ֱ����ɲ����գ����տɵ�ԭ���ʵ���____________![]() �����
�����![]() ��
��
![]() һ������ϡ
һ������ϡ![]() ��Һ��������Zn��Ӧ��Ϊ����������
��Һ��������Zn��Ӧ��Ϊ����������![]() ���ʵ��ֲ�Ӱ��
���ʵ��ֲ�Ӱ��![]() ���������ɲ�ȡ�Ĵ�ʩ��______
���������ɲ�ȡ�Ĵ�ʩ��______![]() ����ĸ
����ĸ![]() ��
��
A.��![]() ����
����![]() ��
��![]() ����
����![]() ��
��![]() ��Һ
��Һ![]() �Ӱ�ˮ
�Ӱ�ˮ![]() ��
��![]() ��Һ
��Һ
![]() �����£���
�����£���![]() ��Һ�м���
��Һ�м���![]() ��Һ���ɹ۲쵽��������_______________________________________��������Ӧ�����ӷ���ʽΪ______________________________��������������Һ��pHֵ����Ϊ4������Һ��
��Һ���ɹ۲쵽��������_______________________________________��������Ӧ�����ӷ���ʽΪ______________________________��������������Һ��pHֵ����Ϊ4������Һ��![]() ����ҺΪ_____
����ҺΪ_____![]()
![]() ��֪������
��֪������![]() ��
��
![]() ���������Ũ�ȵ���������������Ϻ���Һ�� ____________ �ԣ���Һ��
���������Ũ�ȵ���������������Ϻ���Һ�� ____________ �ԣ���Һ��![]() __________
__________![]() ����
����![]() ����
����![]() ������
������![]() ��
��![]() ��
��![]() ������������
������������![]() �Ĵ���������Ϻ���Һ��__________________�ԣ���Һ��
�Ĵ���������Ϻ���Һ��__________________�ԣ���Һ��![]() ______
______![]() ����
����![]() ����
����![]() ������
������![]() ��
��![]() ��
��
![]() �����£���
�����£���![]() ��Һ�ζ�
��Һ�ζ�![]() ijһԪ��HA��Һ���õζ�������ͼ��
ijһԪ��HA��Һ���õζ�������ͼ��
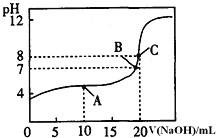
![]() A��B��C������ʾ��Һ����������ǿ���� ______ ���Ӧ����Һ��
A��B��C������ʾ��Һ����������ǿ���� ______ ���Ӧ����Һ��
![]() �������Ũ�ȴ�С��ϵ�� ________________________________ ��
�������Ũ�ȴ�С��ϵ�� ________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������![]() �㷺����ũҩ��ҽҩ����������ҵ�Ʊ��������Ĺ����л��������Ʒ������
�㷺����ũҩ��ҽҩ����������ҵ�Ʊ��������Ĺ����л��������Ʒ������![]() ����ش��������⣺
����ش��������⣺
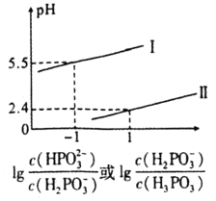
![]() ��֪������
��֪������![]() Ϊ��Ԫ���ᣬ��
Ϊ��Ԫ���ᣬ��![]() ��Һ�У�������Ũ�ȵĴ�С��ϵΪ________��
��Һ�У�������Ũ�ȵĴ�С��ϵΪ________��
![]() �����£���NaOH��Һ�μӵ�������
�����£���NaOH��Һ�μӵ�������![]() ��Һ�У������ҺpH������Ũ�ȱ仯�Ĺ�ϵ��ͼ��ʾ�����ʾ
��Һ�У������ҺpH������Ũ�ȱ仯�Ĺ�ϵ��ͼ��ʾ�����ʾ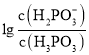 ��������________
��������________![]() ����������������
����������������![]() ��������
��������![]() ��
��![]() ________����Ӧ
________����Ӧ![]() ��ƽ�ⳣ��ֵ��________��
��ƽ�ⳣ��ֵ��________��
![]() ��ҵ��������������ͬʱ���������ˮ
��ҵ��������������ͬʱ���������ˮ![]() ��Ҫ�ɷ�Ϊ
��Ҫ�ɷ�Ϊ![]() ��
��![]() �����ˮ���ȼ�������Ư�ۣ��ټ�����ʯ�ҵ���pH������Ԫ��ת��Ϊ����ĸ��γ��������ա���������ķ�ˮ��
�����ˮ���ȼ�������Ư�ۣ��ټ�����ʯ�ҵ���pH������Ԫ��ת��Ϊ����ĸ��γ��������ա���������ķ�ˮ��![]() ������Һ��
������Һ��![]() ________
________![]() ��
��![]() ��֪
��֪![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾװ�ã�������ָ�뷢��ƫת��ͬʱA����֣�B����ϸ��CΪ�������Һ����A��B��CӦ�����и����е�(����)

A. A��Zn��B��Cu��CΪϡ����
B. A��Cu��B��Zn��CΪϡ����
C. A��Fe��B��Ag��CΪϡAgNO3��Һ
D. A��Ag��B��Fe��CΪϡAgNO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������������ء�����˵��������� ( )
A.ʹ��ҽ�þƾ�ɱ�������Ĺ�����ֻ�����������仯
B.ҽ�ÿ����IJ���ԭ���ϳɷ�֮һ�Ǿ۱�ϩ����ṹ��ʽΪ![]()
C.����һ��Ӧ��ش�ţ��Ա��⵰���ʱ���
D.ҽ�÷������ĺ��IJ��������ķ���ϩ��Ĥ���䵥���ķ���ϩ����±����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ƥा��п�������Ч����Ƥ�ؾ��п������˾���Ч��������һ�������¿ɷ�����ͼ��ʾ��ת��������˵����ȷ����( )

A.����Ƥ�����Ƥ�ػ�Ϊͬϵ��
B.��Ƥ��һ���������ܷ����ӳɷ�Ӧ����ȥ��Ӧ��ȡ����Ӧ
C.1 mol ��Ƥ�������� 3 mol NaOH ��Ӧ
D.ÿ������Ƥष�����������ȫ�ӳɺ�IJ����к��� 5 ������̼ԭ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com