
 ¢Ü¹ļĶé ¢ŻCH3COOH
¢Ü¹ļĶé ¢ŻCH3COOH  ¢ß
¢ß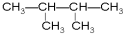 ¢ą
¢ą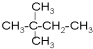 ¢į±ūĶé
¢į±ūĶé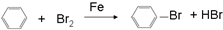 £»
£» £»
£»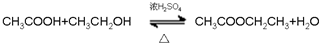 £®
£® ·ÖĪö £Ø1£©øł¾ŻĶéĢžµÄĶØŹ½CnH2n+2£¬øł¾ŻĻą¶Ō·Ö×ÓÖŹĮæĪŖ44ĮŠŹ½¼ĘĖć£»
£Ø2£©øł¾ŻĶéĢžµÄĶØŹ½CnH2n+2£¬½«14øöĒāŌ×Ó“śČėĶØŹ½¼ĘĖć£»
£Ø3£©Ķ¬·ÖŅģ¹¹ĢåŹĒÖø·Ö×ÓŹ½ĻąĶ¬£¬µ«½į¹¹²»Ķ¬µÄ»ÆŗĻĪļ£»
£Ø4£©¾ßÓŠĢŲŹāĘųĪ¶£¬³£×÷ŻĶČ”¼Į£¬øĆÓŠ»śĪļÓėŅŗäå·¢ÉśČ”“ś·“Ó¦£¬ŌņĪŖ±½£¬±½Óėäåµ„ÖŹ·“Ӧɜ³Éäå±½£»
£Ø5£©ŅŅ“¼ÓėCuO·“Ӧɜ³ÉŅŅČ©”¢CuŗĶĖ®£»
£Ø6£©ŅŅĖįÓėŅŅ“¼·¢Éśõ„»Æ·“Ӧɜ³ÉŅŅĖįŅŅõ„ŗĶĖ®£®
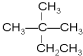
½ā“š ½ā£ŗ£Ø1£©ĶéĢžµÄĶØŹ½ĪŖ£ŗCnH2n+2£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖ44µÄĶéĢž£¬Ōņ12n+2n+2=44£¬ĖłŅŌn=3£¬¼“ĶéĢžµÄ·Ö×ÓŹ½ĪŖC3H8£¬ĪŖ¢į±ūĶ飬½į¹¹¼ņŹ½ĪŖCH3CH2CH3£¬
¹Ź“š°øĪŖ£ŗCH3CH2CH3£»
£Ø2£©ĶéĢžµÄ·Ö×ÓÖŠŗ¬ÓŠ14øöĒāŌ×Ó£¬ĶéĢžµÄĶØŹ½ĪŖ£ŗCnH2n+2£¬Ōņ2n+2=14£¬ĖłŅŌn=6£¬¼“ĶéĢžµÄ·Ö×ÓŹ½ĪŖC6H14£¬ĪŖ¼ŗĶ飬¼“¢Ū¢ß¢ą£¬
¹Ź“š°øĪŖ£ŗC6H14£»
£Ø3£©¢Ū¢ß·Ö×ÓŹ½ĻąĶ¬£¬½į¹¹²»Ķ¬£¬»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬¢ŪÓė¢ąĪŖĶ¬Ņ»ÖÖĪļÖŹ£¬
¹Ź“š°øĪŖ£ŗ¢ß£»
£Ø4£©¾ßÓŠĢŲŹāĘųĪ¶£¬³£×÷ŻĶČ”¼ĮµÄÓŠ»śĪļĪŖ±½£¬ŌŚĢś×÷“߻ƼĮµÄĢõ¼žĻĀÓėŅŗäå·¢ÉśŅ»Č”“ś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ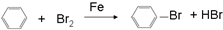 £¬
£¬
¹Ź“š°øĪŖ£ŗ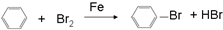 £»
£»
£Ø5£©ŅŅ“¼ÓėCuO·“Ӧɜ³ÉŅŅČ©”¢CuŗĶĖ®£¬·“Ó¦ĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø6£©ŅŅĖįÓėŅŅ“¼·¢Éśõ„»Æ·“Ó¦£¬ĖįĶŃōĒ»ł“¼ĶŃĒā£¬Éś³ÉŅŅĖįŅŅõ„ŗĶĖ®£¬øĆ·“Ó¦ĪŖ£ŗ £¬
£¬
¹Ź“š°øĪŖ£ŗ £®
£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄ½į¹¹ÓėŠŌÖŹ£¬°ŃĪÕ¹ŁÄÜĶÅÓėŠŌÖŹµÄ¹ŲĻµ”¢ÓŠ»ś·“Ó¦ĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪöÓėÓ¦ÓĆÄÜĮ¦µÄ漲飬×ŪŗĻŠŌ½ĻĒ棬ĢāÄæÄŃ¶Č²»“ó£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
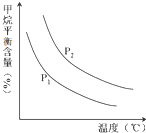 ŗĻ³É°±¼¼ŹõµÄ““Į¢æŖ±ŁĮĖČĖ¹¤¹ĢµŖµÄÖŲŅŖĶ¾¾¶£¬ĘäŃŠ¾æĄ“×ŌÕżČ·µÄĄķĀŪÖøµ¼£¬ŗĻ³É°±·“Ó¦µÄĘ½ŗā³£ŹżKÖµŗĶĪĀ¶ČµÄ¹ŲĻµČēĻĀ£ŗ
ŗĻ³É°±¼¼ŹõµÄ““Į¢æŖ±ŁĮĖČĖ¹¤¹ĢµŖµÄÖŲŅŖĶ¾¾¶£¬ĘäŃŠ¾æĄ“×ŌÕżČ·µÄĄķĀŪÖøµ¼£¬ŗĻ³É°±·“Ó¦µÄĘ½ŗā³£ŹżKÖµŗĶĪĀ¶ČµÄ¹ŲĻµČēĻĀ£ŗ| ĪĀ ¶Č£Ø”ę£© | 360 | 440 | 520 |
| KÖµ | 0.036 | 0.010 | 0.0038 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H2ŗĶD2»„ĪŖĶ¬Ī»ĖŲ | B£® | 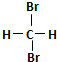 ŗĶ ŗĶ 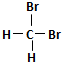 »„ĪŖĶ¬·ÖŅģ¹¹Ģå »„ĪŖĶ¬·ÖŅģ¹¹Ģå | ||
| C£® | Õż¶”ĶéŗĶŅģ¶”ĶéŹĒĶ¬ĻµĪļ | D£® | 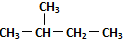 ŗĶ ŗĶ 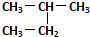 ŹĒĶ¬Ņ»ÖÖĪļÖŹ ŹĒĶ¬Ņ»ÖÖĪļÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ¼× | ŅŅ | ±ū |
| čÖ×Ó | »Ę¹Ļ | Ęź³Č |
| HOCH2COOH | C3H4O5 | 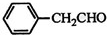 |
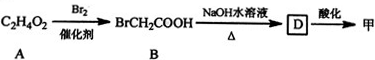
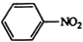 $\stackrel{Fe£¬HCl}{”ś}$
$\stackrel{Fe£¬HCl}{”ś}$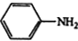 $”ś_{£Ø2£©H_{2}O£¬H+£¬”÷}^{£Ø1£©NaNO_{2}£¬HCl}$
$”ś_{£Ø2£©H_{2}O£¬H+£¬”÷}^{£Ø1£©NaNO_{2}£¬HCl}$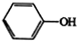
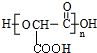 +£Øn-1£©H2O£®
+£Øn-1£©H2O£®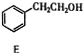 $\stackrel{¾Į½²½}{”ś}$
$\stackrel{¾Į½²½}{”ś}$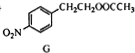 $”ś_{£Ø2£©H+£¬”÷}^{£Ø1£©Fe£¬HCl}$$\stackrel{{C}_{8}{H}_{11}NO}{H}$$”ś_{£Ø2£©H_{2}O£¬H+£¬”÷}^{£Ø1£©NaNO_{2}£¬HCl}$J
$”ś_{£Ø2£©H+£¬”÷}^{£Ø1£©Fe£¬HCl}$$\stackrel{{C}_{8}{H}_{11}NO}{H}$$”ś_{£Ø2£©H_{2}O£¬H+£¬”÷}^{£Ø1£©NaNO_{2}£¬HCl}$J²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
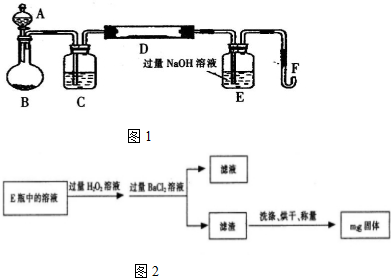
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
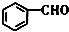 µÄĆū³ĘŹĒ±½¼×Č©£®
µÄĆū³ĘŹĒ±½¼×Č©£®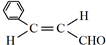 £¬·Ö×ÓÖŠ×ī¶ąÓŠ18øöŌ×Ó¹²Ę½Ćę£®
£¬·Ö×ÓÖŠ×ī¶ąÓŠ18øöŌ×Ó¹²Ę½Ćę£® £®
£®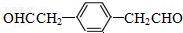 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
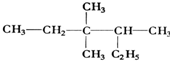 3£¬3£¬4-Čż¼×»ł¼ŗĶé
3£¬3£¬4-Čż¼×»ł¼ŗĶé 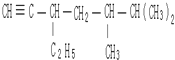 5£¬6-¶ž¼×»ł-3-ŅŅ»ł-1-øżČ²
5£¬6-¶ž¼×»ł-3-ŅŅ»ł-1-øżČ²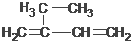 2-ŅŅ»ł-1£¬3-¶”¶žĻ©
2-ŅŅ»ł-1£¬3-¶”¶žĻ© 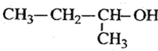 2-¶”“¼
2-¶”“¼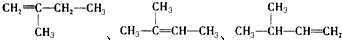 £®
£®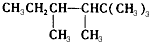 £¬ČōAŹĒµ„Ļ©ĢžÓėĒāĘų¼Ó³ÉŗóµÄ²śĪļ£®ŌņøƵ„Ļ©ĢžæÉÄÜÓŠ5ÖÖ½į¹¹£®
£¬ČōAŹĒµ„Ļ©ĢžÓėĒāĘų¼Ó³ÉŗóµÄ²śĪļ£®ŌņøƵ„Ļ©ĢžæÉÄÜÓŠ5ÖÖ½į¹¹£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚŗĻ³É°±µÄ¹¤ŅµÉś²śÖŠ£¬Ź¹ÓĆ½ĻøßĪĀ¶ČÓŠĄūÓŚĢįøß²śĮæ | |
| B£® | ŌŚŗĻ³É°±µÄ¹¤ŅµÉś²śÖŠ£¬¼ÓŃ¹ÓŠĄūÓŚĢįøß°±µÄ²śĮæ | |
| C£® | ľĢæ·ŪĖéŗóÓėO2·“Ó¦£¬ĖŁĀŹøüæģ | |
| D£® | ÓÉH2£Øg£©”¢I2£Øg£©”¢HIĘųĢå×é³ÉµÄĘ½ŗāĢåĻµ¼ÓŃ¹ŗóŃÕÉ«±äÉī |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com