
����Ϊ�ı�δ�������ʮ���¿Ƽ�֮һ��ȼ�ϵ�ؾ�������Ⱦ������������Ч�ʵ��ص㣮��ͼΪ����ȼ�ϵ�صĽṹʾ��ͼ���������ҺΪKOH��Һ���缫����Ϊ���ɶ��ʯī�����������������ֱ��������ϵش�����������ͨ��ȼ�ϵ��ʱ������ڱպϻ�·�в��ϵز����������Իش��������⣺
��1�� д������ȼ�ϵ�ع���ʱ�����缫��Ӧ����ʽ��
___________ ��
��2�����������ȼ�ϵ��ÿת��0.1mol���ӣ����ı�״����_______L������
��3�� ������ȼ�ϵ�ظĽ�Ϊֱ���Լ��������Ϊԭ�Ͻ��й���ʱ��������ӦʽΪ_______�� ��������ӷ�Ӧ����ʽΪ___________________________��
��1��2H2O��O2��4e��=4OH�� ��2�֣�
��2��0.56L��2�֣�
��3��CH4��10OH����8e��=CO32-��7H2O ��2�֣�
CH4��2O2��2OH��=CO32-��3H2O��2�֣�
���������������Ϊ����ȼ�ϵ�صĵ������ҺΪ���ԣ�����������ӦʽΪ2H2O��O2��4e��=4OH������ÿת��1mol���ӣ����ı�״��������5.6L������ת��0.1mol����ʱ�����ı�״��������0.56L,���Լ���Ϊȼ��ʱ������Ϊ������Ӧ��缫��ӦʽΪCH4��10OH����8e��=CO32-��7H2O��������ӦʽΪ2O2+4H2O+8e-=8OH-,�����缫��Ӧʽ�ϲ�Ϊ��ص��ܷ�ӦCH4��2O2��2OH��=CO32-��3H2O��
���㣺ԭ��ط�Ӧ����ʽ����д����ؼ��㡣


 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣���ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ����������£�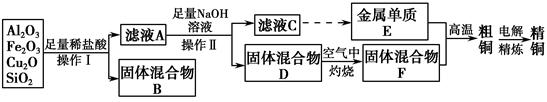
��֪��Cu2O��2H��=Cu��Cu2����H2O
��1��ʵ������������Ϊ________���ڿ��������չ�������Dʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����________�����������ƣ���
��2����ҺA����Ԫ�صĴ�����ʽΪ________�������ӷ��ţ������ɸ����ӵ����ӷ���ʽΪ_______________________________________________
________��������ҺA�д��ڸ����ӵ��Լ�Ϊ________�����Լ����ƣ���
��3����������E���������F������ijһ��Ӧ�����ں��Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________��
��4�������£���pH��NaAlO2��NaOH������Һ�У���ˮ�������c��OH����ǰ��Ϊ���ߵ�108������������Һ��pH��________��
��5�������õ�ⷨ���д�ͭ����ʱ������������ȷ����________������ţ���
a������ȫ��ת��Ϊ��ѧ��
b����ͭ�ӵ�Դ����������������Ӧ
c����ͭ��������������Һ��Cu2��Ũ�ȼ�С
d����ͭ����ʱͨ���ĵ�������������ͭ������ȷ����ϵ
�ڴ�Ũ���ᡢŨ���ᡢ����ˮ��ѡ�ú��ʵ��Լ����ⶨ��ͭ��Ʒ�н���ͭ�������������漰����Ҫ���裺��ȡһ����������Ʒ��______________�����ˡ�ϴ�ӡ����������ʣ�����ͭ������������ȱ�ٵIJ������裬���������������̵�ϸ�ڣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ҵ���������ɡ���������ȡ������ұ�������ļӹ��Ȼ��ڹ��ɡ���ش��������⣺
��1����ҵ�ϲ��õ���������ͱ���ʯ(Na3AlF6)������ķ���ұ���õ���������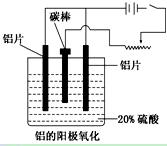
2Al2O3  4Al��3O2��
4Al��3O2��
�������ʯ�����ã�________________________________________________��
��2��������������������������������Fe��Si�����ʣ����õ�ⷽ����һ���ᴿ���õ����������ĵ缫��ӦʽΪ________________�����п����������ϵ���__________��
A������ B��ʯī C��Ǧ�� D������
��3������������ʹ���������������ܵ�����Ĥ����ϡ����Ϊ���Һ�������������ĵ缫��ӦʽΪ_____________________________________________________________��
��4�������������������У���Ҫ���ϵص�����ѹ��������_________________��
��5������˵����ȷ����__________________��
A������������Ӧ��ԭ���ԭ�����н������ϱ��洦���ļ���
B������������������ǿ������ľ�Ե����
C������������������߽���������Ͻ����ʴ�ԣ�����ĥ���½�
D��������������Ĥ���ж���ԣ����к�ǿ���������ܣ�������Ⱦ�϶��ʸ�����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������ɫ�ͳ����µ�Ksp���±���ʾ��
| | Cu(OH)2 | CuOH | CuCl | Cu2O |
| ��ɫ | ��ɫ | ��ɫ | ��ɫ | ש��ɫ |
| Ksp(25 ��) | 1��6��10��19 | 1��0��10��14 | 1��2��10��6 | �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ�ֿ��Է�����硢�ŵ��װ�á���һ�������ڳ��ͷŵ�ʱ�����ķ�ӦΪNiO2+Fe+2H2O Fe(OH)2+Ni(OH)2
Fe(OH)2+Ni(OH)2
��1�������طŵ�ʱ��������ԭ��Ӧ�������� ������ĸ����ͬ����
A��NiO2 B��Fe C��Fe(OH)2 D��Ni(OH)2
��2�������йظõ�ص�˵������ȷ����
A���ŵ�ʱ�������Һ��ǿ����
B���ŵ�ʱ5.6g Feȫ��ת��ΪFe(OH)2ʱ�����·��ͨ����0.2 mol����
C�����ʱ������ӦΪNi(OH)2+2OH��?2e��==NiO2+2H2O
D�����ʱ����������Һ�ļ��Ա��ֲ���
��3���ô����ص�⺬��0.01 mol CuSO4��0.01 mol NaCl�Ļ����Һ100 mL�����صĵ缫��Ϊ���Ե缫������Һ�е�Cu2+ ȫ��ת����Cuʱ�����������������ڱ�״���µ����Ϊ �����������Һ��ˮϡ����1L����ʱ��Һ��pH= ��
��4���ô����ؽ��е�⣬�ҵ��صĵ缫��Ϊͭ�缫���������ҺΪŨ��Һ��NaCl��Һ�Ļ��Һ�����һ��ʱ���ͬѧ�Ǿ���ط��֣�������������������ɫ�������������ɺ�ɫ�������������ϵ�֪�ú�ɫ������Cu2O��д���������ϵĵ缫��Ӧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺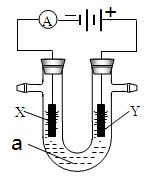
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��ӦʽΪ ����X�������۲쵽�������� ��
��Y�缫�ϵĵ缫��ӦʽΪ ������õ缫��Ӧ����ķ����� ��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ����� ���缫��Ӧʽ�� ��
��Y�缫�IJ����� ���缫��Ӧʽ�� ��
��˵�������ʷ����ĵ缫��Ӧ����д����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ����գ�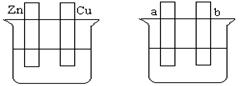
A B
��1����Aͼ�У�ϡ����Ϊ�������Һ���õ������Ӻ�ͭƬ�缫��Ӧʽ ��
��2����Bͼ�����ֱ����Դ����Ҫ��a����ͭ�����Ա�Ҫ�����Ӻ�װ�ý� ��b���缫��Ӧʽ ��
��3����Bͼ�����ֱ����Դ�����缫Ϊ���Ե缫���������Һ��CuSO4��Һ��������������ܷ�Ӧ���ӷ���ʽΪ ����������3.2 g���������Ϸų��������ڱ�״���µ������_____L������һ������ ���ѧʽ������Һ�ָܻ�������ǰ��ȫһ�¡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��l������ijЩ�����������ڸ����µķ�Ӧ��Ϊ���ȷ�Ӧ��������ұ�����۵������
��֪��4Al��s����3O2��g��=2Al2O3��s��  ��-2830kJ��mol-1
��-2830kJ��mol-1
 =+230kJ��mol-1
=+230kJ��mol-1
 =-390kJ��mol-1
=-390kJ��mol-1
�����������������ȷ�Ӧ���Ȼ�ѧ����ʽ�� ��
��2������ͼ��ʾ����������ʢ�к�ˮ���������б���ʴʱ�ɿ쵽����˳���� ��
��װ����Cu�缫�ϵĵ缫��ӦʽΪ ��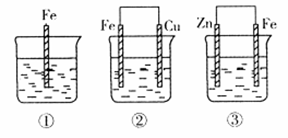
��3������V�����仯����㷺Ӧ��������Դ����ȫ��Һ�����ܵ�������ò�ͬ��̬���ӶԵ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã���ԭ����ͼ��ʾ��
�ٵ������Һ���ɻƱ�������缫��ӦʽΪ ��
�ڳ������У��Ҳ���Һ��ɫ���� ɫ��Ϊ ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��10�֣�ij��ѧ��ȤС���ͬѧΪ��̽�����缫�ڵ���е�����,��Ʋ�����������һϵ��ʵ��,ʵ������¼����:
| ��� | �缫���� | �������Һ | ������ָ��ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��C(ʯī) | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | ����������Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com