

���� ��1����������ǿ�������Σ�������ˮ�����ɴ�����������ƣ�
��2����������Һ�д��������ˮ�����ɴ��ᣬ������Һ�д��������Ũ����Сֱ���ﵽƽ��״̬����Һ��PH������ֱ�����䣬ˮ�����ӻ��������������������䣻
��3������Ӧ���Ũ�Ȼ��С������Ũ�ȣ���ˮ�ⷴӦ������Ӧ�����ƶ���
��4���ڴ�������Һ�м����������������Һ�д��������غ㡢����غ㣬�ݴ˽��
��5������m=CVM�������ʵ�������
��6������ˮ��ƽ�ⳣ��K=$\frac{{K}_{w}}{Ka��C{H}_{3}COOH��}$���м��㣮
��� �⣺��1��������ˮ�����ɴ�����������ƣ���ѧ����ʽΪCH3COONa+H2O?CH3COOH+NaOH���ʴ�Ϊ��CH3COONa+H2O?CH3COOH+NaOH��
��2��A�������Ӳ�ˮ�⣬����Ũ��ʼ�ղ��䣬��A����
B����������ӿ�ʼʱˮ�����������С��ƽ��ʱ���ٱ仯����B��ȷ��
C������ˮ������У�pH������ƽ��ʱ���ٱ仯����C��ȷ��
D��KW��һ�¶ȳ������¶Ȳ��䣬KW���䣬��D����
��ѡBC��
��3��A�������������Һ�д���Ũ������ƽ�����ƣ���A����
B�����봿����壬��ƽ����ϵ������Ũ����Ӱ�죬ƽ�ⲻ�ƶ�����B����
C���������ƹ��壬��Һ�ڴ��������Ũ������ƽ�����ƣ���C��ȷ��
D�������Ȼ�粒��壬笠�������ˮ�����ɵ����������ӽ�ϳ�һˮ�ϰ���ʹ��Һ������������Ũ�ȼ�С��ƽ�����ƣ���D��ȷ��
��ѡCD��
��4��A����������ᣬʹ���������Ũ������������Ũ�Ȳ��䣬���Դ���c��CH3COO-��+c��CH3COOH����c��Na+������A��ȷ��
B���������������ᣬƽ�����ƣ����������Ũ��������������Ũ�ȣ���B����
C����������ᣬ����Һ�д���Ũ�Ƚϴ�ʱ������ĵ�����ڴ�������ӵ�ˮ��̶ȣ����������Ũ��������Һ�����ԣ���C��ȷ��
D�������Ƿ����̶ȴ���ˮ��̶ȣ����������c��OH-����c��Na+������D����
��ѡAC��
��5������һ����m=nM=CVM=0.175mol/L��0.5L��82g/mol=7.175g������������ƽ����������Ϊ7.2g��
���������������������Ƶ�Ũ�ȵ������ϣ���Ϻ����ҺŨ�ȼ���Ϊ0.175mol/L������ԭ����Ũ��Ϊ0.35mol/L��
�ʴ�Ϊ��7.2��0.35mol/L��
��6��ˮ��ƽ�ⳣ��K=$\frac{{K}_{w}}{Ka��C{H}_{3}COOH��}$=$\frac{c��{H}^{+}��•c��O{H}^{-}��}{\frac{c��{H}^{+}��•c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}}$=$\frac{{c}^{2}��O{H}^{-}��}{c��C{H}_{3}CO{O}^{-}��}$=$\frac{1{0}^{-14}}{1.75��1{0}^{-5}}$��C��OH-��=10-5 mol/L������Һ��pH=9���ʴ�Ϊ��9��
���� ���⿼����ۺϣ��漰ˮ��ƽ�ⳣ���ļ��㡢����ˮ��ƽ���ƶ�������жϵ�֪ʶ�㣬�ѵ���ˮ��ƽ�ⳣ����ʽ�����任��ע�⣨5����������ƽ�ĸ�����Ϊ�״��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
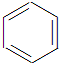 +HNO3$��_{��}^{Ũ����}$
+HNO3$��_{��}^{Ũ����}$ +H2O
+H2O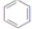 +3H2$��_{��}^{Ni}$
+3H2$��_{��}^{Ni}$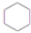
| A�� | �ڢۢݢ� | B�� | �٢ڢ� | C�� | �ܢݢޢ� | D�� | �ڢۢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢܢ� | B�� | �ڢܢ� | C�� | �ڢۢ� | D�� | ���϶�����ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���³�ѹ�£�14g��N2��CO��ɵĻ�����庬�е�ԭ����ĿΪNA | |
| B�� | ��״���£�22.4L���Ậ��NA��HCl���� | |
| C�� | ���³�ѹ�£�8g O2����4NA������ | |
| D�� | ���³�ѹ�£�22.4L��NO2��CO2������庬��2nA��Oԭ�� | |
| E�� | ���³�ѹ�£�18 g H2O �к��е�ԭ������Ϊ3NA | |
| F�� | ��״���£�11.2 L CH3CH2OH �к��еķ�����ĿΪ0.5NA | |
| G�� | ��������ΪNA��NO2��CO2��������к��е���ԭ����Ϊ2NA | |
| H�� | 28g��ϩ�ͻ����飨C4H8���Ļ�������к��е�̼ԭ����Ϊ2NA | |
| I�� | ��״���£�22.4L��CCl4�к��е�CCl4������ΪNA | |
| J�� | 18g H2O�к���������Ϊ10NA | |
| K�� | 46g NO2��N2O4��������к���ԭ������Ϊ3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�11.2L��ȩ�����е�����Ϊ8NA | |
| B�� | 25��ʱ��pH=12��NaCN��Һ��ˮ�����H+��Ϊ10-12NA | |
| C�� | 1L0.5mol•L-1��CHCOOH��Һ���������������ͷ�������Ϊ0.5NA | |
| D�� | 10mol•L-1100mL��Ũ����������ͭ��Ӧ������õ��ĵ�����Ϊ0.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� NH4 ��2SO4 | B�� | NaHCO3 | C�� | Ba Cl2 | D�� | Cu SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KNO3��KClO3�뵥��S��C2H5OH�������ͬһ�ⷿ�� | |
| B�� | �����ơ����軯���Ż�ʱ��ֱ���ø�ѹˮǹ��ˮ���� | |
| C�� | ȼ�������Ӿ������Գ�ζ������������ʣ����Ծ�ʾ�����й© | |
| D�� | �綾��NaCN��Һй©ʱ��ֱ�ӽ��������ˮ����������Ȼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʯ��ˮ�м�������CaO���ָ���ԭ�¶Ⱥ���Һ��pH���� | |
| B�� | ϡ�����ˮϡ�ͣ��������̶�������Һ��pH��С | |
| C�� | �����еμӰ�ˮ�����ԣ���Һ�е�����ֻ���Ȼ�� | |
| D�� | ����ʱpH=3�������pH=11�İ�ˮ�������Ϻ���Һ��pHС��7 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com