
��ͼ��ʵ����������������װ�á�
��1���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ����Һ�ķ�����_________________________________Ȼ����������Թܣ�ʹ֮��Ͼ��ȡ�
��2��Ũ�����������______________��_____________��
��3������Na2CO3��Һ��������___________________________
���ܷ���NaOH��Һ���棬������
��
��4��ʵ�����ɵ��������������ܶȱ�ˮ___ (���С��)����_____��ζ��
��5����ʵ�����¶ȹ��ߣ�ʹ��Ӧ�¶ȴﵽ140������ʱ�������ĸ���Ӧ�ķ���ʽ��______________________________________ _��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A��������һ��������ʵ���ҵ��������ʱ�����뾡�����ռ�����������
B����pH�ơ��絼����(һ�ֲ�����Һ��������������)���ɼ������������ˮ��̶�
C���ڱ���������ؿ����ڱ궨NaOH��Һ��Ũ�ȣ���������ڱ����������ʱ������ƽ������ʵ������ƫ�����õ�NaOH��ҺŨ�ȱ�ʵ��Ũ��ƫС
D����ij��Һ�м�������ͪ�Լ���������к���Һ��������ɫ������жϸ���Һ���е�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йؽ���ұ����˵���У�����ȷ����
A���õ�������Ȼ�þ�ķ���ұ������þ
B���ڼ������������������ԭ�����������õ�������
C�������ȷ������۵�ϸߵĽ�����
D��ֱ�Ӽ����������õ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʽC4H8O2���л�����������Һ���ȿɵ��л���A��B����A�������տɵ�C����B��CΪͬϵ���C�ɷ���������Ӧ����ԭ�л���Ľṹ��ʽΪ��( )
A��HCOOCH2CH2CH3 ��B��CH3COOCH2CH3 C��CH3CH2COOCH3 D��HCOOCH��CH3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȩ������ͱ�ȩ��ɵĻ�����У���Ԫ�ص�����������37%����̼Ԫ�ص���������Ϊ( )
A.27% B.28% C.54% D.������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)1 mol KHSO4�����ۻ��ɵ����2NA������ (����)
(2)���ۻ���������ˮ�������ڹ��ۼ����ƻ�����������ˮ�������ڹ��ۼ������ƻ�
(����)
(3)���ۻ������۵㶼�������ӻ����� (����)
(4)�����ڹ��ۼ�Խǿ������Խ�ȶ������ۡ��е�ҲԽ�� (����)
(5)���������ӵĻ�����һ������������ (����)
(6)�������Ӽ������ʲ������ǵ��� (����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� (����)
A��KOH�мȺ������Ӽ�Ҳ���й��ۼ����������ӻ�����
B��N2���ڵ��ʣ������ڻ�ѧ��
C��MgCl2�мȺ������Ӽ����ֺ��й��ۼ�
D��NH4Cl�к��й��ۼ�����ȫ���ɷǽ���Ԫ����ɣ����ڹ��ۻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪R2����b�����ӣ�������a�����ӣ���ʾRԭ�ӷ�����ȷ����(����)
A.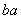 R B.
R B.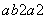 R C.
R C.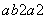 R D��a��ba��2R
R D��a��ba��2R
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com