
·ÖĪö ŅŃÖŖĘųĢåBæÉŹ¹ŹŖČóŗģÉ«ŹÆČļŹŌŅŗ±äĄ¶£¬BŹĒ°±Ęų£¬2.40gNaHµÄĪļÖŹµÄĮæĪŖ0.1molŗĶ°±ĘųB·“Ӧɜ³É3.90g»ÆŗĻĪļAŗĶ0.1molH2¼“ĪŖ0.2g£¬øł¾ŻÖŹĮæŹŲŗć£¬²ĪÓė·“Ó¦µÄ°±ĘųµÄÖŹĮæĪŖ1.7g£¬¼“ĪŖ0.1mol£¬øł¾ŻĢāŅā0.1molNaH+0.1molNH3=0.1molH2+A£¬øł¾ŻÖŹĮæŹŲŗćŌņA»ÆѧŹ½ĪŖNaNH2£¬ĘäĦ¶ūÖŹĮæĪŖ39g/mol£¬Ōņ3.90gNaNH2µÄĪļÖŹµÄĮæĪŖ0.1mol£¬·ūŗĻĢāŅā£¬ÓÉ“Ė·ÖĪö½ā“š£®
½ā“š ½ā£ŗŅŃÖŖĘųĢåBæÉŹ¹ŹŖČóŗģÉ«ŹÆČļŹŌŅŗ±äĄ¶£¬BŹĒ°±Ęų£¬2.40gNaHµÄĪļÖŹµÄĮæĪŖ0.1molŗĶ°±ĘųB·“Ӧɜ³É3.90g»ÆŗĻĪļAŗĶ0.1molH2¼“ĪŖ0.2g£¬øł¾ŻÖŹĮæŹŲŗć£¬²ĪÓė·“Ó¦µÄ°±ĘųµÄÖŹĮæĪŖ1.7g£¬¼“ĪŖ0.1mol£¬øł¾ŻĢāŅā0.1molNaH+0.1molNH3=0.1molH2+A£¬øł¾ŻÖŹĮæŹŲŗćŌņA»ÆѧŹ½ĪŖNaNH2£¬ĘäĦ¶ūÖŹĮæĪŖ39g/mol£¬Ōņ3.90gNaNH2µÄĪļÖŹµÄĮæĪŖ0.1mol£¬
£Ø1£©AµÄ»ÆѧŹ½ŹĒNaNH2£¬¹Ź“š°øĪŖ£ŗNaNH2£»
£Ø2£©NaHÓėĘųĢåB·“Ӧɜ³É»ÆŗĻĪļAµÄ»Æѧ·½³ĢŹ½£ŗNaH+NH3=NaNH2+H2£¬¹Ź“š°øĪŖ£ŗNaH+NH3=NaNH2+H2£»
£Ø3£©AŹĒ×ćĮæŃĪĖį·¢Éś·ĒŃõ»Æ»¹Ō·“Ó¦µÄ»Æѧ·½³ĢŹ½NaNH2+2HCl=NaCl+NH4Cl£¬¹Ź“š°øĪŖ£ŗNaNH2+2HCl=NaCl+NH4Cl£»
£Ø4£©ŌŚøßĪĀĻĀ£ØNaH£©æɽ«ĖÄĀČ»ÆīŃ£ØTiCl4£©»¹Ō³É½šŹōīŃ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2NaH+TiCl4=Ti+2NaCl+2HCl£¬¹Ź“š°øĪŖ£ŗ2NaH+TiCl4=Ti+2NaCl+2HCl£»
£Ø5£©ŅņĪŖŃĒĮņĖįŃĪ¾ßÓŠ¼«ĒæµÄ»¹ŌŠŌ£¬ĪüŹÕŅŗ”±ĶعżÕō·¢½į¾§ÖĘµĆµÄ¹ĢĢåæÉÄÜŹĒŃĒĮņĖįŃĪŗĶĮņĖįŃĪµÄ»ģŗĻĪļ£¬¹Ź“š°øĪŖ£ŗ²»ŗĻĄķ£¬ĶعżÕō·¢½į¾§ÖĘµĆµÄ¹ĢĢåæÉÄÜŹĒŃĒĮņĖįŃĪŗĶĮņĖįŃĪµÄ»ģŗĻĪļ£¬ĖłŅŌµĆµ½µÄ¹ĢĢå²»Ņ»¶ØŹĒ“æ¾»Īļ£¬æÉÄÜŹĒŃĒĮņĖį±µŗĶĮņĖį±µµÄ»ģŗĻĪļ£®
µćĘĄ ±¾Ģāæ¼²éÖŖŹ¶µć½Ļ¶ą£¬»Æѧ·½³ĢµÄŹéŠ“£¬ÖŹĮæŹŲŗć¶ØĀɵÄÓ¦ÓĆ”¢Ńõ»Æ»¹Ō·“Ó¦·½³ĢŹ½µÄÅäĘ½”¢ŌŖĖŲ»ÆŗĻĪļµÄŠŌÖŹµČ£¬ŹōÓŚĘ“ŗĻŠĶĢāÄ棬ŠčŅŖѧɜ¾ß±øŌśŹµµÄ»ł“”£¬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
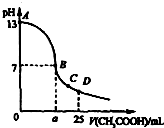 25”ꏱ£¬ŌŚ25mL 0.1mol•L-1µÄNaOHČÜŅŗÖŠ£¬ÖšµĪ¼ÓČė0.2mol•L-1µÄCH3COOHČÜŅŗ£®ČÜŅŗpHµÄ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾£®ĻĀĮŠ·ÖĪöµÄ½įĀŪÖŠ£¬²»ÕżČ·µÄŹĒ£Ø””””£©
25”ꏱ£¬ŌŚ25mL 0.1mol•L-1µÄNaOHČÜŅŗÖŠ£¬ÖšµĪ¼ÓČė0.2mol•L-1µÄCH3COOHČÜŅŗ£®ČÜŅŗpHµÄ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾£®ĻĀĮŠ·ÖĪöµÄ½įĀŪÖŠ£¬²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | CµćŹ±c£ØCH3COO-£©£¾c£ØNa+£©£¾c£ØH+£©£¾c£ØOH-£© | |
| B£® | DµćŹ±c£ØCH3COO-£©+c£ØCH3COOH£©=2c£ØNa+£© | |
| C£® | ĒśĻßÉĻA”¢B¼äČĪŅ»µć£¬ČÜŅŗÖŠ¶¼ÓŠ£ŗc£ØNa+£©£¾c£ØCH3COO-£©£¾c£ØOH-£©£¾c£ØH+£© | |
| D£® | BµćµÄŗį×ų±źa=12.5ml |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ŗĶéÓėäåµÄČ”“ś·“Ó¦±ŲŠėŌŚ¹āÕÕĢõ¼žĻĀ²ÅÄܽųŠŠ | |
| B£® | ĀČŅŅĻ©¾ŪŗĻ³É¾ŪĀČŅŅĻ©ĖÜĮĻµÄ±¾ÖŹŹĒ¼Ó³É·“Ó¦ | |
| C£® | ±½ÓėäåµÄČ”“ś·“Ó¦µÄ“߻ƼĮæÉŅŌŹĒFeBr3£¬Ņ²æÉŅŌŹĒFe·Ū | |
| D£® | äåŅŅĶéµÄÖĘČ”²ÉČ”¼Ó³É·“Ó¦»ņČ”“ś·“Ó¦µÄ·½·Ø¶¼Ņ»Ńł |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
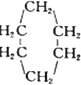 æɱķŹ¾ĪŖ
æɱķŹ¾ĪŖ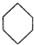 £¬ĻĀĆęŹĒijøß¾ŪĪļµÄŗĻ³ÉĀ·Ļߣ¬ŹŌĶź³ÉĻĀĮŠŹŌĢā£ŗ
£¬ĻĀĆęŹĒijøß¾ŪĪļµÄŗĻ³ÉĀ·Ļߣ¬ŹŌĶź³ÉĻĀĮŠŹŌĢā£ŗ $”ś_{¢ń}^{NH}$
$”ś_{¢ń}^{NH}$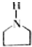 $”ś_{¢ņ}^{CH=CH}$A$\stackrel{¢ó}{”ś}$
$”ś_{¢ņ}^{CH=CH}$A$\stackrel{¢ó}{”ś}$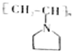
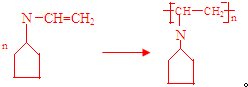 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH3CH2CH2CH3 | B£® | CH3CH£ØCH3£©2 | C£® | CH3C£ØCH3£©3 | D£® | £ØCH3£©2CHCH2CH3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | nCH2ØTCH2-”ś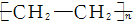 | B£® | CH2ØTCH2+HCl-”śCH3CH2Cl | ||
| C£® | 2CH3CH2OH+O2$”ś_{”÷}^{Cu}$2CH2CHO+2H2O | D£® | 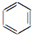 +Br2$\stackrel{FeBr_{3}}{”ś}$ +Br2$\stackrel{FeBr_{3}}{”ś}$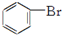 +HBr +HBr |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻĖĪ¬ĖŲ | B£® | µ°°×ÖŹ | C£® | ¾ŪŅŅĻ© | D£® | µķ·Ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
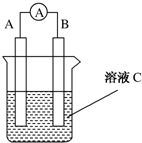 ČēĶ¼ĖłŹ¾ŹĒŌµē³ŲµÄ×°ÖĆĶ¼£Ø
ČēĶ¼ĖłŹ¾ŹĒŌµē³ŲµÄ×°ÖĆĶ¼£Ø ĪŖµēĮ÷±ķ£©£®Ēė»Ų“š£ŗ
ĪŖµēĮ÷±ķ£©£®Ēė»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com