
”¾ĢāÄæ”æI£®Ä³¹¤³§·ĻĖ®ÖŠŗ¬ÓĪĄėĢ¬ĀČ£¬ĶعżĻĀĮŠŹµŃé²ā¶ØĘäÅØ¶Č”£
¢ŁČ”Ė®Ńł10.0 mLӌ׶ŠĪĘæÖŠ£¬¼ÓČė10.0 mLµÄKIČÜŅŗ(×ćĮæ)£¬·¢ÉśµÄ·“Ó¦ĪŖ£ŗCl2+2KI£½2KCl+I2£¬µĪČėÖøŹ¾¼Į2”«3µĪ”£
¢ŚČ”Ņ»µĪ¶Ø¹ÜŅĄ“ĪÓĆ×ŌĄ“Ė®”¢ÕōĮóĖ®Ļ“¾»ŗó£¬ŌŁÓĆ0.01mol”¤L-1 Na2S2O3ČÜŅŗČóĻ“£¬Č»ŗó×°Čė0.01mol”¤L-1 Na2S2O3ČÜŅŗµ½0æĢ¶ČŅŌÉĻ£¬ÅųöĻĀ¶Ė¼ā×ģÄŚµÄĘųÅŻ£¬µ÷ÕūŅŗĆęÖĮ0æĢ¶Č»ņ0æĢ¶ČĻĀijŅ»Ī»ÖĆ£¬¼ĒĻĀ¶ĮŹż”£
¢Ū½«×¶ŠĪĘæÖĆÓŚµĪ¶Ø¹ÜĻĀ½ųŠŠµĪ¶Ø£¬·¢ÉśµÄ·“Ó¦ĪŖ£ŗI2+2Na2S2O3=2NaI+ 2Na2S4O6”£ŹŌ»Ų“šĻĀĮŠĪŹ“š£ŗ
£Ø1£©²½Öč¢Ł¼ÓČėµÄÖøŹ¾¼ĮŹĒ_______________________________”£
£Ø2£©²½Öč¢ŚÓ¦Ź¹ÓĆ________Ź½µĪ¶Ø¹Ü”£
£Ø3£©ÅŠ¶Ļ“ļµ½µĪ¶ØÖÕµćµÄŹµŃéĻÖĻóŹĒ___________________________________”£
¢ņ£®£Ø4£©ČōÓĆ0.1032 mol/L HClČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄNaOHČÜŅŗ£¬ĻĀĮŠĒéæö¶ŌŹµŃé½į¹ūĪŽÓ°ĻģµÄŹĒ____________”£
A.ĖįŹ½µĪ¶Ø¹ÜĪ“ÓƱź×¼ŃĪĖįČÜŅŗČóĻ“
B.׶ŠĪĘæĪ“ÓĆ“ż²āŅŗČóĻ“
C.µĪ¶ØĒ°µĪ¶Ø¹Ü¼ā×ģÖŠÓŠŅ»ĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§ĮĖ
D.µĪ¶ØŹ±½«±ź×¼Ņŗ½¦³ö׶ŠĪĘæĶā
£Ø5£©Ģ¼ĖįH2CO3£¬K1=4.3”Į10-7£¬K2=5.6”Į10-11£¬²ŻĖįH2C2O4 K1=5.9”Į10-2£¬K2=6.4”Į10-5”£0.1 mol/L Na2CO3ČÜŅŗµÄpH____________0.1 mol/L Na2C2O4ČÜŅŗµÄpH(Ń”Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)”£Čō½«µČÅØ¶ČµÄ²ŻĖįČÜŅŗŗĶĢ¼ĖįČÜŅŗµČĢå»ż»ģŗĻ£¬ČÜŅŗÖŠø÷ÖÖĄė×ÓÅØ¶Č“óŠ”µÄĖ³ŠņÕżČ·µÄŹĒ_____________”£
A£®c(H+)£¾c(HC2O4-)£¾c(HCO3-)£¾c£ØCO32-)
B£®c(HCO3-)£¾c(HC2O4-)£¾c(C2O42-)£¾c(CO32-)
C£®c(H+)£¾c(HC2O4-)£¾c(C2O42-)£¾c(CO32-)
D£®c(H2CO3) £¾c(HCO3-)£¾c(HC2O4-)£¾c(CO32-)
”¾“š°ø”æµķ·ŪČÜŅŗ¼īµĪČė×īŗóŅ»µĪ±ź×¼Ņŗ£¬ČÜŅŗÓÉĄ¶É«±ä³ÉĪŽÉ«ĒŅ°ė·ÖÖÓÄŚ²»»Öø“B“óÓŚAC
”¾½āĪö”æ
(1)ČÜŅŗÖŠÓŠµ„ÖŹµā£¬¼ÓČėµķ·ŪČÜŅŗ³ŹĄ¶É«£¬µāÓėŃĒĮņĖįÄĘ·¢ÉśŃõ»Æ»¹Ō·“Ó¦£¬µ±·“Ó¦ÖÕµćŹ±£¬Ą¶É«ĶŹČ„£»¹Ź“š°øĪŖ£ŗµķ·ŪČÜŅŗ£»
(2)Įņ“śĮņĖįÄĘČÜŅŗĻŌ¼īŠŌ£¬Ó¦Ń”Ōń¼īŹ½µĪ¶Ø¹Ü£»¹Ź“š°øĪŖ£ŗ¼ī£»
(3)µāÓöµķ·Ū±äĄ¶É«£¬ĖłŅŌČÜŅŗ³ŹĄ¶É«£¬Ėę·“Ó¦I2+2Na2S2O3=2NaI+2Na2S4O6½ųŠŠ£¬ČÜŅŗ֊ƻӊµā£¬ČÜŅŗÓÉĄ¶É«ĪŖĪŽÉ«£¬ĖµĆ÷·“Ó¦µ½ÖÕµć£¬ÅŠ¶Ļ“ļµ½µĪ¶ØÖÕµćµÄŹµŃéĻÖĻóŹĒ£ŗµĪ×īŗóŅ»µĪČÜŅŗ£¬ÓÉĄ¶É«Ē”ŗƱäĪŖĪŽÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»±äÉ«£»¹Ź“š°øĪŖ£ŗµĪ×īŗóŅ»µĪČÜŅŗ£¬ÓÉĄ¶É«Ē”ŗƱäĪŖĪŽÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»±äÉ«£»
(4)A”¢ĖįŹ½µĪ¶Ø¹ÜĪ“ÓƱź×¼ŃĪĖįČÜŅŗČóĻ“£¬±ź×¼ŃĪĖįµÄÅضČĘ«Š”£¬Ōģ³ÉV(±ź×¼)Ę«“ó£¬øł¾Żc(“ż²ā)=![]() æÉÖŖ£¬²ā¶Øc(“ż²ā)Ę«“󣬹ŹA“ķĪó£»B”¢×¶ŠĪĘæĪ“ÓĆ“ż²āŅŗČóĻ“£¬¶ŌV(±ź×¼)ĪŽÓ°Ļģ£¬øł¾Żc(“ż²ā)=
æÉÖŖ£¬²ā¶Øc(“ż²ā)Ę«“󣬹ŹA“ķĪó£»B”¢×¶ŠĪĘæĪ“ÓĆ“ż²āŅŗČóĻ“£¬¶ŌV(±ź×¼)ĪŽÓ°Ļģ£¬øł¾Żc(“ż²ā)=![]() æÉÖŖ£¬²ā¶Øc(“ż²ā)ĪŽÓ°Ļģ£¬¹ŹBÕżČ·£»C”¢µĪ¶ØĒ°µĪ¶Ø¹Ü¼ā×ģÖŠÓŠŅ»ĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§ĮĖ£¬Ōģ³ÉV(±ź×¼)Ę«“ó£¬øł¾Żc(“ż²ā)=
æÉÖŖ£¬²ā¶Øc(“ż²ā)ĪŽÓ°Ļģ£¬¹ŹBÕżČ·£»C”¢µĪ¶ØĒ°µĪ¶Ø¹Ü¼ā×ģÖŠÓŠŅ»ĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§ĮĖ£¬Ōģ³ÉV(±ź×¼)Ę«“ó£¬øł¾Żc(“ż²ā)=![]() æÉÖŖ£¬²ā¶Øc(“ż²ā)Ę«“󣬹ŹC“ķĪó£»D”¢µĪ¶ØŹ±½«±ź×¼Ņŗ½¦³ö׶ŠĪĘæĶā£¬Ōģ³ÉV(±ź×¼)Ę«Š”£¬øł¾Żc(“ż²ā)=
æÉÖŖ£¬²ā¶Øc(“ż²ā)Ę«“󣬹ŹC“ķĪó£»D”¢µĪ¶ØŹ±½«±ź×¼Ņŗ½¦³ö׶ŠĪĘæĶā£¬Ōģ³ÉV(±ź×¼)Ę«Š”£¬øł¾Żc(“ż²ā)=![]() æÉÖŖ£¬²ā¶Øc(“ż²ā)Ę«Š”£¬¹ŹD“ķĪó£»¹ŹŃ”B£»
æÉÖŖ£¬²ā¶Øc(“ż²ā)Ę«Š”£¬¹ŹD“ķĪó£»¹ŹŃ”B£»
(5)²ŻĖįµÄ¶ž¼¶µēĄė³£ŹżµČÓŚĢ¼ĖįµÄ¶ž¼¶µēĄė³£Źż£¬ĖµĆ÷²ŻĖįĒāøłµÄĖįŠŌ±ČĢ¼ĖįĒāøłµÄĒ棬Ōņ0.1 mol/L Na2CO3ČÜŅŗÖŠĢ¼ĖįøłµÄĖ®½ā³Ģ¶Č“óÓŚ0.1 mol/L Na2C2O4ČÜŅŗÖŠ²ŻĖįøłµÄĖ®½ā³Ģ¶Č£¬¹Ź0.1 mol/L Na2CO3ČÜŅŗ¼īŠŌøüĒ棬¼“0.1mol/LNa2CO3ČÜŅŗµÄpH“óÓŚ0.1mol/LNa2C2O4ČÜŅŗµÄpH£¬²ŻĖįµÄŅ»¼¶”¢¶ž¼¶µēĄė³£Źż¾ł“óÓŚĢ¼ĖįµÄŅ»¼¶µēĄė³£Źż£¬²ŻĖį”¢Ģ¼ĖįµÄŅ»¼¶µēĄėŌ¶“óÓŚ¶ž¼¶µēĄė£¬µŚŅ»²½µēĄėĪŖÖ÷£¬Ņņ“ĖČÜŅŗÖŠc (H+)£¾c (HC2O4-)£¾c (C2O42-)£¾c (HCO3-)£¾c (CO32-)£¬ŌņACÕżČ·£¬BD“ķĪ󣬹Ź“š°øĪŖ£ŗ“óÓŚ£»AC”£


 »ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
»ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”潫ĻĀĶ¼ĖłŹ¾ŹµŃé×°ÖĆµÄ K ±ÕŗĻ,ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ
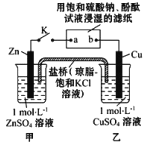
A£®Ę¬æĢŗó¼×³ŲÖŠ c(SO42-£©Ōö“ó B£®µē×ÓŃŲ Zn”śa”śb”śCu Ā·¾¶Į÷¶Æ
C£®Cu µē¼«ÉĻ·¢Éś»¹Ō·“Ó¦ D£®Ę¬æĢŗóæɹŪ²ģµ½ĀĖÖ½ b µć±äŗģÉ«
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¢ń.ÓĆNaOHČÜŅŗĪüŹÕŃĢĘųÖŠµÄSO2£¬½«ĖłµĆµÄNa2SO3ČÜŅŗ½ųŠŠµē½ā£¬æÉŃ»·ŌŁÉśNaOH£¬Ķ¬Ź±µĆµ½H2SO4£¬ĘäŌĄķČēĶ¼ĖłŹ¾(µē¼«²ÄĮĻĪŖŹÆÄ«)”£

(1)Ķ¼ÖŠa¼«ŅŖĮ¬½ÓµēŌ“µÄ_____(Ģī”°Õż”±»ņ”°øŗ”±)¼«£¬SO![]() ·ÅµēµÄµē¼«·“Ó¦_____________”£
·ÅµēµÄµē¼«·“Ó¦_____________”£
¢ņ.ŌŚČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ£¬ČōĶØČėÖ±Į÷µē5 minŹ±£¬Ķµē¼«ÖŹĮæŌö¼Ó2.16 g£¬ŹŌ»Ų“š£ŗ
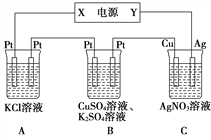
(2)ČÜŅŗpH±ä»Æ£ŗ B________£¬C________(Ģī”°Ōö“ó”±”°¼õŠ””±»ņ”°²»±ä”±)”£
(3)Ķصē5 minŹ±£¬BÖŠ¹²ŹÕ¼Æ224 mLĘųĢå(±ź×¼×“æö)£¬ČÜŅŗĢå»żĪŖ200 mL”£ŌņĶصēĒ°CuSO4ČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ________(Éčµē½āĒ°ŗóČÜŅŗĢå»żĪŽ±ä»Æ)”£
(4)ČōAÖŠ×ćĮæµÄKClČÜŅŗµÄĢå»żŅ²ŹĒ200 mL£¬µē½āŗó£¬ČÜŅŗµÄpHĪŖ_____(Éčµē½āĒ°ŗóČÜŅŗĢå»żĪŽ±ä»Æ)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”潫Na2O2·Ö±šĶ¶ČėĻĀĮŠĪļÖŹµÄĖ®ČÜŅŗÖŠ£¬Éś³É°×É«³ĮµķµÄŹĒ£Ø £©
A. BaCl2 B. K2SO4 C. CuCl2 D. Ca(HCO3)2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
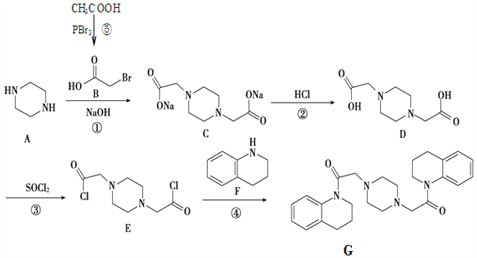
”¾ĢāÄæ”æĪļÖŹGµÄŗĻ³ÉĀ·ĻßČēĶ¼£ŗ
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĪļÖŹBÖŠ¹ŁÄÜĶŵÄĆū³ĘĪŖ__________________£®
£Ø2£©Š“³ö¢Ł·“Ó¦ĄąŠĶ£ŗ__________________£®
£Ø3£©Š“³ö²½Öč¢ŪµÄ·“Ó¦·½³ĢŹ½_____________________________________£®
£Ø4£©Čē¹ūƻӊ²½Öč¢Ū£¬DŗĶFÄÜ·“Ó¦Āš£æ______£®
£Ø5£©ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ___________£®
A £®AŹĒ·¼Ļć×å»ÆŗĻĪļ
B £®DæÉŅŌ·¢Éśõ„»Æ·“Ó¦
C £®F ÖŠĖłÓŠŌ×Ó¹²Ę½Ćę
D £®GæÉŅŌÓė·¢Éś¼Ó³É·“Ó¦
£Ø6£©Ķ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄFµÄĶ¬·ÖŅģ¹¹ĢåÓŠ______Ö֣ز»ŗ¬Į¢Ģå½į¹¹£©£®
¢Łŗ¬ÓŠC=C¼ü
¢Śŗ¬ÓŠ±½»·ĒŅĘäÖ»ÓŠĮ½øöČ”“ś»ł
¢Ūŗ¬ÓŠ©¤NH2
Š“³öĘäÖŠŗĖ“Ź²ÕńĻŌŹ¾ÓŠ5ÖÖĒāŌ×ÓµÄĖłÓŠĪļÖŹµÄ½į¹¹¼ņŹ½£ŗ_____________________________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ³żŌÓĖłŃ”ÓƵďŌ¼Į¼°²Ł×÷·½·Ø¾łÕżČ·µÄŅ»×éŹĒ(ĄØŗÅÄŚĪŖŌÓÖŹ)
Ń”Ļī | “żĢį“æµÄĪļÖŹ | Ń”ÓƵďŌ¼Į | ²Ł×÷·½·Ø |
A | NaHCO3(Na2CO3) | ŹŹĮæŃĪĖį | Õō·¢½į¾§ |
B | CO2(CO) | O2 | µćČ¼ |
C | Mg(Al) | ĒāŃõ»ÆÄĘČÜŅŗ | ¹żĀĖ |
D | CO2(HCl) | ĒāŃõ»ÆÄĘČÜŅŗ | Ļ“Ęų |
A. A B. B C. C D. D
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠø÷×éĪļÖŹ»ģŗĻŗ󣬲»ÄÜÉś³ÉNaOHµÄŹĒ
A.NaŗĶH2OB.Ca(OH)2ČÜŅŗŗĶNaClČÜŅŗ
C.Na2O2ŗĶH2OD.Ca(OH)2ČÜŅŗŗĶNa2CO3ČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµ£¬ĻĀĮŠŠšŹö²»ÕżČ·µÄŹĒ
A. ³£ĪĀ³£Ń¹ĻĀ£¬1.7 g°±ĘųÖŠŗ¬ÓŠµÄŌ×ÓŹżÄæĪŖ0.4NA
B. 50 mL 1 mol”¤L1 K2SO4ČÜŅŗÖŠŗ¬ÓŠµÄK+ŹżÄæĪŖ0.1NA
C. 5.6 gĢśÓė×ćĮæĻ”ĮņĖį·“Ó¦×ŖŅʵĵē×ÓŹżĪŖ0.3NA
D. ±ź×¼×“æöĻĀ£¬4.48 LµÄŃõĘųŗĶµŖĘųµÄ»ģŗĻĪļŗ¬ÓŠµÄ·Ö×ÓŹżÄæĪŖ0.2NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÉčŌŚČŻ»żæɱäµÄĆܱÕČŻĘ÷ÖŠ³äČė10 mol N2(g)ŗĶ10 mol H2(g)£¬·“Ó¦ŌŚŅ»¶ØĢõ¼žĻĀ“ļµ½Ę½ŗāŹ±£¬NH3µÄĢå»ż·ÖŹżĪŖ0.25”£¼ĘĖć[£Ø1£©”¢£Ø2£©µÄ¼ĘĖć¶¼Šč°“øńŹ½Š“¼ĘĖć¹ż³Ģ£¬·ńŌņ“š°ø¶ŌŅ²²»øų·Ö]
£Ø1£©øĆĢõ¼žĻĀ·“Ó¦N2(g)+3H2(g)![]() 2NH3(g) µÄĘ½ŗā³£Źż________”££ØÉčøĆĢõ¼žĻĀ£¬Ćæ1molĘųĢåĖłÕ¼µÄĢå»żĪŖVL£©
2NH3(g) µÄĘ½ŗā³£Źż________”££ØÉčøĆĢõ¼žĻĀ£¬Ćæ1molĘųĢåĖłÕ¼µÄĢå»żĪŖVL£©
£Ø2£©ÉĻŹö·“Ó¦µÄĘ½ŗāŹ±£¬ŌŁ³äČė10 molµÄN2£¬øł¾Ż¼ĘĖć£¬Ę½ŗāÓ¦ĻņŹ²Ć“·½ĻņŅʶÆ________
£Ø3£©Ä³ĪĀ¶ČĻĀ½«ĖłµĆµÄ°±ĘųÅä³É0.1 molL-1µÄČÜŅŗ£¬ĖłµĆČÜŅŗµÄpOH_____£»½«øĆČÜŅŗ³åĻ”100±¶£»¼ĘĖć“ĖŹ±°±Ė®µÄµēĄė¶ČĪŖ________(ŅŃÖŖøĆĪĀ¶ČĻĀKb(NH3”¤H2O) =1.0”Į10-5)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com