2014������������������Ű�ҹ��ж�����������ǿ����β���ŷż���ȼú��ҵ���������ڼ�������������Ҫ�����壮

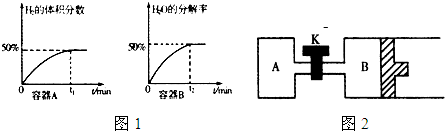
��1������ԭ��ع���ԭ���ⶨ����β����CO��Ũ�ȣ���װ����ͼ1��ʾ���õ���е����Ϊ����̼���Σ�CO
32-�ڹ�������п��������ƶ�������AΪ
�����������ĵ缫��Ӧʽ
��
��2��ij��ѧ��ȤС������ͼ��ʾ���̣��ⶨ��״�������ΪV L��ȼú������SO
2�����
��

���SO
2���������Ϊ
���ú���V��m�Ĵ���ʽ��ʾ����
��3����֪������������ʵĵ���ƽ�ⳣ������
| NH3?H2O | Kb=1.8��10-5mol?L-1 |
| H2SO3 | Ka1=1.2��10-2mol?L-1 Ka2=1.3��10-8mol?L-1 |
�����ð�ˮ���չ�ҵ�����е�SO
2��������Һʧȥ��������ʱ�����ʱ��Һ��
�ԣ���ᡱ������С�����
����ͨ������Ĺ����У�ˮ�ĵ���̶���α仯����ǡ���γ�����ʱ����Һ������Ũ�ȵĴ�С��ϵΪ
��
�ۿ�ͨ����ⷨʹ����Һ������ѭ�����ã��缫��Ϊʯī�缫���������ɻ���ԭ�����ᣮ�乤��ʾ��ͼ��ͼ2����д����������ĵ缫��Ӧʽ
��





 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
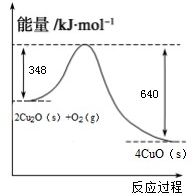 ��ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����
��ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����