

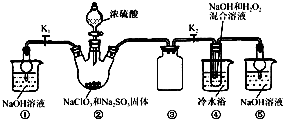
ͼ1-21
������������⣺
(1)���β���������ָ��Ϊʲô�ᷢ��ƫת��______________��
(2)���β���������ָ��ƫת����Ϊʲô�෴�����û�ѧƽ���ƶ�ԭ�����͡�
(3)�ٲ���������C1���Ϸ����ķ�ӦΪ____________________________��
(4)�ڲ���������C2���Ϸ����ķ�ӦΪ____________________________��
������![]() +2I-+2H+
+2I-+2H+![]()
![]() +I2+H2O�ǿ��淴Ӧ��ƽ���ƶ������ܡ�c(H+)��Ӱ�졣����HCl��ƽ������������NaOHƽ���涯������ԭ��ط�Ӧ�����ķ����෴��������ָ���෴��
+I2+H2O�ǿ��淴Ӧ��ƽ���ƶ������ܡ�c(H+)��Ӱ�졣����HCl��ƽ������������NaOHƽ���涯������ԭ��ط�Ӧ�����ķ����෴��������ָ���෴��
��HClʱ�������ĵ缫��ӦΪ��2I-![]() I2+2e-
I2+2e-
�����ĵ缫��ӦΪ�� ![]() +2H++2e-
+2H++2e-![]()
![]() +H2O
+H2O
��NaOH �����ĵ缫��ӦΪ��![]() +H2O
+H2O![]()
![]() +2H++2e-
+2H++2e-
�����ĵ缫��ӦΪ��I2+2e-![]() 2I-
2I-
�𰸣�(1)��ʵ�������»�ѧ��ת��Ϊ����
(2)����������Ũ�ȵĸı䣬ʹ��ѧƽ����ͬ�����ƶ�����������˲�ͬ����ķ�Ӧ��
(3)2I-![]() I2+2e-
I2+2e-
(4)![]() +H2O
+H2O![]()
![]() +2H++2e-
+2H++2e-


 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| O | - 2 |
| O | - 2 |
| O | 2- 6 |
| 90.5cV |
| 4m |
| 90.5cV |
| 4m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�Իش��������⣺
(1)���β���������ָ��Ϊʲô�ᷢ��ƫת?________________________________________��
(2)���β���������ָ��ƫת����Ϊʲô�෴?���û�ѧƽ���ƶ�ԭ�����͡�____________________________��
(3)�ٲ���������C1���Ϸ����ķ�ӦΪ______________________________________________��
(4)�ڲ���������C2���Ϸ����ķ�ӦΪ______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(��)��(B)�ձ�����μ���Ũ���ᣬ���������ָ��ƫת��
(��)������(B)�ձ��еμ�40%NaOH��Һ���������ָ����ǰ���෴����ƫת���Իش�
(1)���β���������ָ��Ϊʲô�ᷢ��ƫת��
(2)���β���������ָ��ƫת����Ϊʲô���෴������ƽ���ƶ�ԭ�����ʹ�����
(3)(��)����������C1���Ϸ����ķ�ӦΪ_____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com