
”¾ĢāÄæ”æŗ¬µŖ»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śÖŠ¶¼ÓŠÖŲŅŖÓ¦ÓĆ”£
£Ø1£©°±ŗĶėĀ£ØN2H4£©ŹĒĮ½ÖÖ×ī³£¼ūµÄµŖĒā»ÆĪļ”£
¼ŗÖŖ£ŗ4NH3(g)£«3O2(g) ![]() 2N2(g)£«6H2O(g) ¦¤H1=£541.8kJ/mol£¬»ÆŃ§Ę½ŗā³£ŹżĪŖK1”£N2H4(g)£«O2(g)
2N2(g)£«6H2O(g) ¦¤H1=£541.8kJ/mol£¬»ÆŃ§Ę½ŗā³£ŹżĪŖK1”£N2H4(g)£«O2(g) ![]() N2(g)£«2H2O(g) ¦¤H2=£534kJ/mol£¬»ÆŃ§Ę½ŗā³£ŹżĪŖK2”£ŌņÓĆNH3ŗĶO2ÖĘČ”N2H4µÄČČ»Æѧ·½³ĢŹ½ĪŖ_________£¬øĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£ŹżK=____£ØÓĆK1”¢K2±ķŹ¾£©”£
N2(g)£«2H2O(g) ¦¤H2=£534kJ/mol£¬»ÆŃ§Ę½ŗā³£ŹżĪŖK2”£ŌņÓĆNH3ŗĶO2ÖĘČ”N2H4µÄČČ»Æѧ·½³ĢŹ½ĪŖ_________£¬øĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£ŹżK=____£ØÓĆK1”¢K2±ķŹ¾£©”£
£Ø2£©¶ŌÓŚ2NO(g)£«2CO(g) ![]() N2(g)£«2CO2(g)£¬ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬ÓŚ1LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ³äČė0.1molNOŗĶ0.3molCO£¬·“Ó¦æŖŹ¼½ųŠŠ”£
N2(g)£«2CO2(g)£¬ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬ÓŚ1LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ³äČė0.1molNOŗĶ0.3molCO£¬·“Ó¦æŖŹ¼½ųŠŠ”£
¢ŁĻĀĮŠÄÜĖµĆ÷øĆ·“Ó¦ŅŃ¾“ļµ½Ę½ŗāדĢ¬µÄŹĒ____£ØĢī×ÖÄø“śŗÅ£©”£
A£®c(CO)=c(CO2) B£®ČŻĘ÷ÖŠ»ģŗĻĘųĢåµÄĆÜ¶Č²»±ä
C£®v(N2)Õż=2v(NO)Äę D£®ČŻĘ÷ÖŠ»ģŗĻĘųĢåµÄĘ½¾łÄ¦¶ūÖŹĮæ²»±ä
¢ŚĶ¼1ĪŖČŻĘ÷ÄŚµÄŃ¹Ēæ£ØP£©ÓėĘšŹ¼Ń¹Ēæ£ØP0£©µÄ±ČÖµ£ØP/P0£©Ėꏱ¼ä£Øt£©µÄ±ä»ÆĒśĻß”£0”«5minÄŚ£¬øĆ·“Ó¦µÄĘ½¾ł·“Ó¦ĖŁĀŹv(N2)= ____£¬Ę½ŗāŹ±NOµÄ×Ŗ»ÆĀŹĪŖ____”£

£Ø3£©Ź¹ÓĆ¼ä½Óµē»Æѧ·ØæÉ“¦ĄķČ¼ĆŗŃĢĘųÖŠµÄNO£¬×°ÖĆČēĶ¼2ĖłŹ¾”£ŅŃÖŖµē½ā³ŲµÄŅõ¼«ŹŅÖŠČÜŅŗµÄpHŌŚ4”«7Ö®¼ä£¬Š“³öŅõ¼«µÄµē¼«·“Ó¦Ź½_________”£ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ĪüŹÕ³ŲÖŠ³żČ„NOµÄŌĄķ__________________”£
”¾“š°ø”æ 4NH3(g)£«O2(g) = 2N2H4(g)£«2H2O(g) ¦¤H=£«526.2kJ/mol ![]() D 0.006 mol”¤L£1”¤min£1 80% 2HSO3££«2e££«2H£«=S2O42££«2H2O 2NO£«2S2O42££«2H2O=N2£«4HSO3£
D 0.006 mol”¤L£1”¤min£1 80% 2HSO3££«2e££«2H£«=S2O42££«2H2O 2NO£«2S2O42££«2H2O=N2£«4HSO3£
”¾½āĪö”æ(1)¢Ł4NH3(g)£«3O2(g) ![]() 2N2(g)£«6H2O(g) ¦¤H1=£541.8kJ/mol£¬»ÆŃ§Ę½ŗā³£ŹżĪŖK1”£¢ŚN2H4(g)£«O2(g)
2N2(g)£«6H2O(g) ¦¤H1=£541.8kJ/mol£¬»ÆŃ§Ę½ŗā³£ŹżĪŖK1”£¢ŚN2H4(g)£«O2(g) ![]() N2(g)£«2H2O(g) ¦¤H2=£534kJ/mol£¬»ÆŃ§Ę½ŗā³£ŹżĪŖK2”£øł¾ŻøĒĖ¹¶ØĀÉ£¬½«¢Ł-¢Ś”Į2µĆ£ŗ4NH3(g)£«O2(g) = 2N2H4(g)£«2H2O(g) ¦¤H=(£541.8kJ/mol)-(£534kJ/mol)”Į2=£«526.2kJ/mol£¬øĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£ŹżK=
N2(g)£«2H2O(g) ¦¤H2=£534kJ/mol£¬»ÆŃ§Ę½ŗā³£ŹżĪŖK2”£øł¾ŻøĒĖ¹¶ØĀÉ£¬½«¢Ł-¢Ś”Į2µĆ£ŗ4NH3(g)£«O2(g) = 2N2H4(g)£«2H2O(g) ¦¤H=(£541.8kJ/mol)-(£534kJ/mol)”Į2=£«526.2kJ/mol£¬øĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£ŹżK=![]() £¬¹Ź“š°øĪŖ£ŗ4NH3(g)£«O2(g) = 2N2H4(g)£«2H2O(g) ¦¤H=£«526.2kJ/mol£»
£¬¹Ź“š°øĪŖ£ŗ4NH3(g)£«O2(g) = 2N2H4(g)£«2H2O(g) ¦¤H=£«526.2kJ/mol£» ![]() £»
£»
(2)¶ŌÓŚ2NO(g)£«2CO(g) ![]() N2(g)£«2CO2(g)£¬ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬ÓŚ1LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ³äČė0.1molNOŗĶ0.3molCO£¬·“Ó¦æŖŹ¼½ųŠŠ”£
N2(g)£«2CO2(g)£¬ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬ÓŚ1LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ³äČė0.1molNOŗĶ0.3molCO£¬·“Ó¦æŖŹ¼½ųŠŠ”£
¢ŁA£®c(CO)=c(CO2)£¬²»±ķŹ¾ÅØ¶Č±ä»Æ£¬²»ÄÜÅŠ¶ĻŹĒ·ńĪŖĘ½ŗāדĢ¬£¬¹ŹA“ķĪó£»B£®·“Ó¦ÖŠĘųĢåµÄÖŹĮæ²»±ä£¬Ģå»ż²»±ä£¬ČŻĘ÷ÖŠ»ģŗĻĘųĢåµÄĆܶȏ¼ÖÕ²»±ä£¬²»ÄÜÅŠ¶ĻŹĒ·ńĪŖĘ½ŗāדĢ¬£¬¹ŹB“ķĪó£»C£®v(N2)Õż=2v(NO)Äę±ķŹ¾£¬Ó¦øĆŹĒ2v(N2)Õż=v(NO)Äę£¬²Å±ķŹ¾ÕżÄę·“Ó¦ĖŁĀŹĻąµČ£¬¹ŹC“ķĪó£»D£®øĆ·“Ó¦ŹōÓŚĘųĢåµÄĪļÖŹµÄĮæ·¢Éś±ä»ÆµÄ·“Ó¦£¬ČŻĘ÷ÖŠ»ģŗĻĘųĢåµÄĘ½¾łÄ¦¶ūÖŹĮæ²»±äŹ±±ķŹ¾ĘųĢåµÄĪļÖŹµÄĮæ²»±ä£¬ ĖµĆ÷ŹĒĘ½ŗāדĢ¬£¬¹ŹDÕżČ·£»¹ŹŃ”D£»
¢Śøł¾ŻČŻĘ÷ÄŚµÄŃ¹Ēæ(P)ÓėĘšŹ¼Ń¹Ēæ(P0)µÄ±ČÖµ(P/P0)Ėꏱ¼ä(t)µÄ±ä»ÆĒśĻߣ¬0”«5minÄŚ£¬ ![]() =0.925£¬øł¾Ż°¢·üŁ¤µĀĀŽ¶ØĀɼ°ĘäĶĘĀŪ£¬
=0.925£¬øł¾Ż°¢·üŁ¤µĀĀŽ¶ØĀɼ°ĘäĶĘĀŪ£¬ ![]() =0.925£¬Ę½ŗāŹ±
=0.925£¬Ę½ŗāŹ±![]() =0.90£¬
=0.90£¬
2NO(g)£« 2CO(g) ![]() N2(g)£«2CO2(g)
N2(g)£«2CO2(g)
ĘšŹ¼(mol) 0.1 0.3 0 0
·“Ó¦ 2x 2x x 2x
5min¼°Ę½ŗā
5minŹ±£¬ ![]() =0.925£¬½āµĆx=0.03mol£¬v(N2)=
=0.925£¬½āµĆx=0.03mol£¬v(N2)= 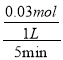 = 0.006mol”¤L£1”¤min£1£»Ę½ŗāŹ±£¬
= 0.006mol”¤L£1”¤min£1£»Ę½ŗāŹ±£¬ ![]() =0.90£¬½āµĆx=0.04mol£¬NOµÄ×Ŗ»ÆĀŹ=
=0.90£¬½āµĆx=0.04mol£¬NOµÄ×Ŗ»ÆĀŹ=![]() ”Į100%=80%£¬¹Ź“š°øĪŖ£ŗ0.006 mol”¤L£1”¤min£1£»80%£»
”Į100%=80%£¬¹Ź“š°øĪŖ£ŗ0.006 mol”¤L£1”¤min£1£»80%£»
(3)Ņõ¼«·¢Éś»¹Ō·“Ó¦£¬ŹĒŃĒĮņĖįĒāøłĄė×Ó£¬µĆµē×Ó£¬Éś³ÉĮņ“śĮņĖįøłĄė×Ó£¬µē¼«·“Ó¦Ź½ĪŖ£ŗ2HSO3-+2e-+2H+ØTS2O42-+2H2O£»Įņ“śĮņĖįøłĄė×ÓÓėŅ»Ńõ»ÆµŖ·¢ÉśŃõ»Æ»¹Ō·“Ó¦£¬Éś³ÉµŖĘų£¬Ąė×Ó·“Ó¦·½³ĢŹ½ĪŖ£ŗ2NO+2S2O42-+2H2OØTN2+4HSO3-£»¹Ź“š°øĪŖ£ŗ2HSO3-+2H++2e-=S2O42-+2H2O£»2NO+2S2O42-+2H2O=N2+4HSO3-”£


 æ¼Ē°±ŲĮ·ĻµĮŠ“š°ø
æ¼Ē°±ŲĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ·ÖĄė·½·Ø²»ŗĻĄķµÄŹĒ( )
A. “ÓŹÆÓĶÖŠµĆµ½ĘūÓĶ£¬æÉÓĆÕōĮóµÄ·½·Ø
B. ĢįČ”äåĖ®ÖŠµÄä壬æÉÓĆ¼ÓČėŅŅ“¼ŻĶČ”µÄ·½·Ø
C. Ö»ŗ¬ÓŠÄąÉ³µÄ“ÖŃĪ£¬æÉĶعżČܽā”¢¹żĀĖ”¢½į¾§µÄ·½·ØĢį“æ
D. ³żFeCl2ČÜŅŗÖŠµÄÉŁĮæFeCl3£¬æÉÓĆ¼ÓČė×ćĮæĢśŠ¼¹żĀĖµÄ·½·Ø
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æX”¢Y”¢Z”¢W”¢M”¢NĪŖŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄĮłÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬³£ĪĀĻĀ£¬ĮłÖÖŌŖĖŲµÄ³£¼ūµ„ÖŹÖŠČżÖÖĪŖĘųĢå£¬ČżÖÖĪŖ¹ĢĢ唣XÓėM£¬WÓėN·Ö±šĶ¬Ö÷×壬ŌŚÖÜĘŚ±ķÖŠXŹĒŌ×Ó°ė¾¶×īŠ”µÄŌŖĖŲ£¬ĒŅXÄÜÓėY”¢Z”¢W·Ö±šŠĪ³Éµē×ÓŹżĻąµČµÄČżÖÖ·Ö×Ó£¬Z”¢WµÄ×īĶā²ćµē×ÓŹżÖ®ŗĶÓėMµÄŗĖĶāµē×Ó×ÜŹżĻąµČ”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)NŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ_____________________£»YµÄ¼ņµ„Ēā»ÆĪļµÄĪČ¶ØŠŌ________(Ģī”°>”±”°<”±»ņ”°£½”±)WµÄ¼ņµ„Ēā»ÆĪļµÄĪČ¶ØŠŌ”£
(2)X”¢ZŠĪ³ÉµÄŗ¬18µē×ӵĻÆŗĻĪļµÄµē×ÓŹ½ĪŖ________________________”£
(3)ÓÉX”¢Z”¢W”¢NĖÄÖÖŌŖĖŲ×é³ÉµÄŅ»ÖÖĄė×Ó»ÆŗĻĪļA£¬ŅŃÖŖA¼ČÄÜÓėŃĪĖį·“Ó¦£¬ÓÖÄÜÓėĀČĖ®·“Ó¦£¬Š“³öAÓė×ćĮæŃĪĖį·“Ó¦µÄĄė×Ó·½³ĢŹ½___________________________________________”£
(4)XŗĶW×é³ÉµÄ»ÆŗĻĪļÖŠ£¬¼Čŗ¬ÓŠ¼«ŠŌ¹²¼Ū¼üÓÖŗ¬ÓŠ·Ē¼«ŠŌ¹²¼Ū¼üµÄŹĒ________(Ģī»ÆѧŹ½)£¬“Ė»ÆŗĻĪļæɽ«¼īŠŌ¹¤Ņµ·ĻĖ®ÖŠµÄCN£Ńõ»Æ£¬Éś³ÉĢ¼ĖįŃĪŗĶ°±Ęų£¬ĻąÓ¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĻÖĻóŅņĪŖ·¢Éś¼Ó³É·“Ó¦¶ų²śÉśµÄŹĒ( )
A£®ŅŅĻ©Ź¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ«
B£®±½ĶØČėäåĖ®ÖŠ£¬Õńµ“ŗóĖ®²ć½Ó½üĪŽÉ«
C£®ŅŅĻ©Ź¹äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗĶŹÉ«
D£®¼×ĶéÓėĀČĘų»ģŗĻ£¬¹āÕÕŅ»¶ĪŹ±¼äŗó»ĘĀĢÉ«ĻūŹ§
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¢ń.Ń”ŌńŗĻŹŹµÄĶ¼Ļó£¬ÓĆ×ÖÄøĢīæÕ£ŗ

£Ø1£©½«µČÖŹĮæµÄĮ½·ŻŠæ·Ūa”¢b·Ö±š¼ÓČė¹żĮæµÄĻ”ĮņĖįÖŠ£¬Ķ¬Ź±ĻņaÖŠ¼ÓČėÉŁĮæµÄCuSO4ČÜŅŗ£¬²śÉśH2µÄĢå»żV(L)ÓėŹ±¼ät(min)µÄ¹ŲĻµµÄĶ¼ĻńŹĒ_______”£
£Ø2£©½«£Ø1£©ÖŠµÄCuSO4ČÜŅŗøijÉCH3COONaČÜŅŗ£¬ĘäĖūĢõ¼ž²»±ä£¬ŌņĶ¼ĻóŹĒ_______”£
¢ņ.Na2FeO4ŹĒŅ»ÖÖ¼ČÄÜɱ¾ś”¢Ļū¶¾£¬ÓÖÄÜŠõÄż¾»Ė®µÄøߊ§Ė®“¦Ąķ¼Į£¬Ęäµē½āÖĘ·ØČēĻĀĶ¼ĖłŹ¾”£µē½ā¹ż³ĢÖŠ£¬Į½¼«¾łÓŠĘųĢå²śÉś£¬Y¼«ĒųÓņČÜŅŗÖš½„±ä³É×ĻŗģÉ«£»Ķ£Ö¹ŹµŃ飬Ģśµē¼«Ć÷ĻŌ±äĻø£¬µē½āŅŗČŌ³ĪĒ唣²éŌÄ׏ĮĻµĆÖŖ£¬øßĢśĖįøłĄė×Ó(FeO42-)ŌŚČÜŅŗÖŠ³Ź×ĻŗģÉ«”£

£Ø3£©µē½ā¹ż³ĢÖŠ£¬X¼«ĒųČÜŅŗµÄpH______(Ģī”°Ōö“ó”±”°¼õŠ””±»ņ”°²»±ä”±)”£
£Ø4£©µē½ā¹ż³ĢÖŠ£¬Y¼«·¢ÉśµÄµē¼«·“Ó¦ĪŖ___________ŗĶ_____________”£
£Ø5£©ČōŌŚX¼«ŹÕ¼Æµ½672 mLĘųĢ壬ŌŚY¼«ŹÕ¼Æµ½168 mLĘųĢå(¾łŅŃÕŪĖćĪŖ±ź×¼×“æöŹ±ĘųĢåĢå»ż)£¬ŌņYµē¼«(Ģśµē¼«)ÖŹĮæ¼õÉŁ__________g”£
£Ø6£©ŌŚ¼īŠŌŠæµē³ŲÖŠ£¬ÓĆøßĢśĖį¼Ų×÷ĪŖÕż¼«²ÄĮĻ£¬µē³Ų·“Ó¦ĪŖ2K2FeO4£«3Zn===Fe2O3£«ZnO£«2K2ZnO2”£øƵē³ŲÕż¼«µÄµē¼«·“Ó¦Ź½ĪŖ______________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¹āĻĖĶØŠÅŹĒŅ»ÖÖĻÖ“ś»ÆµÄĶØŠÅŹÖ¶Ī£¬ÖĘŌģ¹āµ¼ĻĖĪ¬µÄÖ÷ŅŖŌĮĻŹĒ
A. Na2CO3 B. CaO C. CaCO3 D. SiO2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A.ŌŚ¼¦µ°ĒåČÜŅŗÖŠ·Ö±š¼ÓČė±„ŗĶNa2SO4”¢CuSO4ČÜŅŗ£¬¶¼»įŅņŃĪĪö²śÉś³Įµķ
B.Ė¾ÄøĪģ¶¦”¢¶ØŌ¶½¢¼×°å”¢ÓŠ»ś²£Į§µČŌ²ÄĮĻŹōÓŚŗĻ½š
C.¾Ūõ„ĻĖĪ¬”¢Ģ¼ĻĖĪ¬”¢¹āµ¼ĻĖĪ¬¶¼ŹōÓŚÓŠ»śøß·Ö×Ó²ÄĮĻ
D.ŹÆĄÆÓĶŹÜČČ·Ö½ā²śÉśĮĖæÉŅŌŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«µÄĻ©Ģž
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŗŚ»šŅ©µÄ±¬ÕØ·“Ó¦ĪŖ£ŗ2KNO3+3C+S=A+N2”ü+3CO2”ü(ŅŃÅäĘ½)
£Ø1£©³żSŅŌĶā£¬ÉĻŹöĖł¼°ŌŖĖŲµÄµēøŗŠŌ“ӓ󵽊”ŅĄ“ĪĪŖ____________________”£
£Ø2£©ŌŚÉś³ÉĪļÖŠ£¬AµÄµē×ÓŹ½ĪŖ____________£»ŗ¬¼«ŠŌ¹²¼Ū¼üµÄ·Ö×ÓµÄÖŠŠÄŌ×Ó¹ģµĄŌÓ»ÆĄąŠĶĪŖ________”£
£Ø3£©ŅŃÖŖCN-ÓėN2½į¹¹ĻąĖĘ£¬ĶĘĖćHCN·Ö×ÓÖŠ¦Ņ¼üÓė¦Š¼üŹżÄæÖ®±ČĪŖ___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ¶ŌĪļÖŹÓĆĶ¾µÄĆčŹöÖŠ£¬“ķĪóµÄŹĒ£Ø £©
A.ĀĮæÉÓĆÓŚŅ±Į¶Ä³Š©ČŪµć½ĻøߵĽšŹō
B.Na2O2æÉÓĆ×÷ĘÆ°×¼Į
C.¼īŹÆ»ŅæÉÓĆÓŚøÉŌļCO2”¢NH3µČĘųĢå
D.NaClOæÉÓĆ×÷Ļū¶¾¼Į
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com