
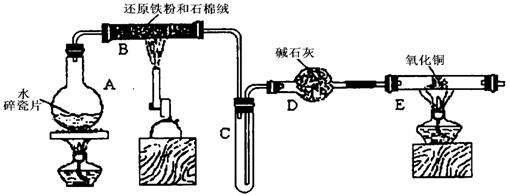
ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��
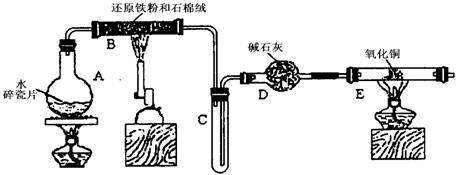
(1) ��Ӳ�ʲ�����B���ȵ�����������_________
(2)B�з�����Ӧ�Ļ�ѧ����ʽ��___________________________________________��
(3)Ϊ�˰�ȫ������E��ǰ������еIJ�����_________________________________��
(4)��֪�з�Ӧ��Cu2O+2H+![]() Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
a��ϡ���� b��ϡ���� c������ d��Ũ����
(5)��E�����ٽӶ���ʢ��ʯ�ҵĸ����(���γ�ΪF��G)�����ø�װ�òⶨˮ����ɻ�ͭ��Ħ������������Ӧǰʢ��ҩƷ��E��F�������ֱ���bg��cg����Ӧ��ֱ���dg��eg��
��ˮ��Ԫ�ص����ʵ���֮�ȿɱ�ʾΪ�����û���n(H)��n(O)= _______�U_______������Ӧ��E�г�Cu��������ֻ�ԭ����Cu2O����ñ�ֵ��___________ (ѡ�ƫ��ƫС������Ӱ�족)��
�����յ�E�ܵ�����Ϊag�����Ⱥ�CuO��ȫ��ԭΪCu����ͭ��Ħ�������ɱ�ʾΪ______________________________��
��6��ijѧ��Ϊ�˼��鷴Ӧ�����ijɷ֣���Ӧǰ��E���м���������ͭ16g����Ӧ����������������Ϊ13.6g����13.6g�ijɷ�Ϊ��____________����Ϊ�������������Ե�������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| b-d |
| 16 |
| b-d |
| 16 |
| 16(d-a) |
| b-d |
| 16(d-a) |
| b-d |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
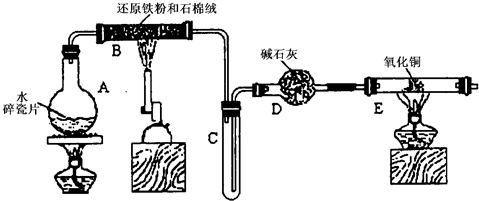
(12��)ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��
(1)Ϊ�˰�ȫ������E��ǰ������еIJ�����____________________________��
(2) B�з�����Ӧ�Ļ�ѧ����ʽ�� _______________��
(3)��֪�з�Ӧ��Cu2O+2H+Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
a��ϡ���� b��ϡ���� c������ d��Ũ����
(4)��E�����ٽӶ���ʢ��ʯ�ҵĸ����(���γ�ΪF��G)�����ø�װ�òⶨˮ����ɡ�����Ӧ��ʢ��ҩƷ��E������������eg��F������������f g��ˮ��Ԫ�ص����ʵ���֮�ȿɱ�ʾΪ�����û���n(H)��n(O)= ________��_______������Ӧ��E�г�Cu�������һ�ֻ�ԭ����Cu2O����ñ�ֵ��___________ (ѡ�ƫ��ƫС������Ӱ�족)��
(5)ijѧ��Ϊ�˼��鷴Ӧ�����ijɷ֣���Ӧǰ��E���м���������ͭ����16g����Ӧ����������������Ϊ13.6g����13.6g�ijɷ�Ϊ��___________����Ϊ�������������Ե�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ӱ�ʡ����һ�и�����ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ�ʵ����
(12��)ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��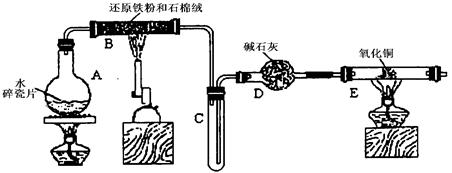
(1)Ϊ�˰�ȫ������E��ǰ������еIJ�����____________________________��
(2) B�з�����Ӧ�Ļ�ѧ����ʽ�� _______________��
(3)��֪�з�Ӧ��Cu2O+2H+ Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
a��ϡ���� b��ϡ���� c������ d��Ũ����
(4)��E�����ٽӶ���ʢ��ʯ�ҵĸ����(���γ�ΪF��G)�����ø�װ�òⶨˮ����ɡ�����Ӧ��ʢ��ҩƷ��E������������e g��F������������f g��ˮ��Ԫ�ص����ʵ���֮�ȿɱ�ʾΪ�����û���n(H)��n(O)= ________��_______������Ӧ��E�г�Cu�������һ�ֻ�ԭ����Cu2O����ñ�ֵ��___________ (ѡ�ƫ��ƫС������Ӱ�족)��
(5)ijѧ��Ϊ�˼��鷴Ӧ�����ijɷ֣���Ӧǰ��E���м���������ͭ����16g����Ӧ����������������Ϊ13.6g����13.6g�ijɷ�Ϊ��___________����Ϊ�������������Ե�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ������ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ�ʵ����
(12��)ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��
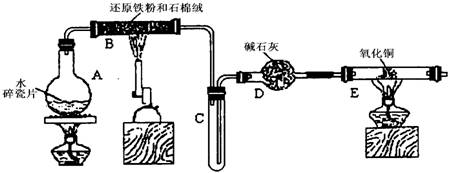
(1)Ϊ�˰�ȫ������E��ǰ������еIJ�����____________________________��
(2) B�з�����Ӧ�Ļ�ѧ����ʽ�� _______________��
(3)��֪�з�Ӧ��Cu2O+2H+ Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
a��ϡ���� b��ϡ���� c������ d��Ũ����
(4)��E�����ٽӶ���ʢ��ʯ�ҵĸ����(���γ�ΪF��G)�����ø�װ�òⶨˮ����ɡ�����Ӧ��ʢ��ҩƷ��E������������e g��F������������f g��ˮ��Ԫ�ص����ʵ���֮�ȿɱ�ʾΪ�����û���n(H)��n(O)= ________��_______������Ӧ��E�г�Cu�������һ�ֻ�ԭ����Cu2O����ñ�ֵ��___________ (ѡ�ƫ��ƫС������Ӱ�족)��
(5)ijѧ��Ϊ�˼��鷴Ӧ�����ijɷ֣���Ӧǰ��E���м���������ͭ����16g����Ӧ����������������Ϊ13.6g����13.6g�ijɷ�Ϊ��___________����Ϊ�������������Ե�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��
ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��
(1) ��Ӳ�ʲ�����B���ȵ�����������_________
(2)B�з�����Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
(3)Ϊ�˰�ȫ������E��ǰ������еIJ�����________________________________________��
(4)��֪�з�Ӧ��Cu20+2H+![]() Cu+Cu2++H20������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu20�����õ��Լ���________(�����)
Cu+Cu2++H20������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu20�����õ��Լ���________(�����)
a��ϡ���� b��ϡ���� c������ d��Ũ����
(5)��E�����ٽӶ���ʢ��ʯ�ҵĸ����(���γ�ΪF��G)�����ø�װ�òⶨˮ����ɻ�ͭ�����ԭ������������Ӧǰʢ��ҩƷ��E��F�������ֱ���bg��cg����Ӧ��ֱ���dg��eg��
��ˮ��Ԫ�ص����ʵ���֮�ȿɱ�ʾΪ�����û���n(H)��n(0)= ________:_______������Ӧ��E�г�Cu��������ֻ�ԭ����Cu20����ñ�ֵ��___________ (ѡ�ƫ��ƫС������Ӱ�족)��
�����յ�E�ܵ�����Ϊag�����Ⱥ�CuO��ȫ��ԭΪCu����ͭ�����ԭ�������ɱ�ʾΪ______________________________��
��6��ijѧ��Ϊ�˼��鷴Ӧ�����ijɷ֣���Ӧǰ��E���м���������ͭ16g����Ӧ����������������Ϊ13.6g����13.6g�ijɷ�Ϊ��____________����Ϊ�������������Ե�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com