
 =0.7mol��
=0.7mol�� =0.6mol��
=0.6mol�� =0.05mol��
=0.05mol�� =0.01mol��
=0.01mol�� =20%��
=20%��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��δ�ʯ���л�ø��������ȼ��һֱ�ǻ�ѧ��̽���Ŀ��⣬��ʯ�ͷ���õ������ͽ����ѻ����Ի�ø��������ȼ�ͣ�
��δ�ʯ���л�ø��������ȼ��һֱ�ǻ�ѧ��̽���Ŀ��⣬��ʯ�ͷ���õ������ͽ����ѻ����Ի�ø��������ȼ�ͣ�
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?��������ģ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
��2013?��������ģ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
| ||
| ||
| �ܽ�ȣ�S��/g | �ܶȻ���Ksp�� | ||
| Ca��OH��2 | Ba��OH��2 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡͬ���� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��δ�ʯ���л�ø��������ȼ��һֱ�ǻ�ѧ��̽���Ŀ��⣬��ʯ�ͷ���õ������ͽ����ѻ����Ի�ø��������ȼ�͡�
����һ��ʯ���Ǻ���20��30��̼ԭ�ӵ������Ļ��������³ʹ�̬��
���϶���ʯ�ʹ��ѻ���ͨ��ʹ��Al2O3��������
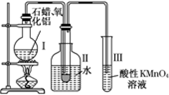
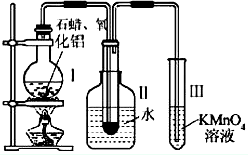
ij�о���ѧϰС����ʵ������ģ��ʯ�͵Ĵ��ѻ���װ����ͼ��ʵ������пɹ۲쵽�ձ����й���ʯ�����ۻ����Թܢ���������Һ�����ᣬ�Թܢ������Ը��������Һ��ɫ��ʵ������Թܢ���Һ����ζ���������͵���ζ��
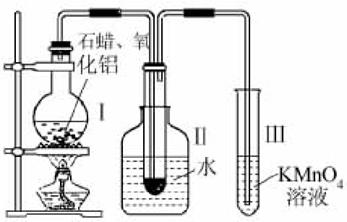
(1)��װ���������ӵ�˳��Ӧ��ѭ��ԭ��Ϊ__________��Ϊ��֤ʵ��ɹ���ʵ��ǰ������еIJ�����__________��װ���нϳ����ܵ�������______________________________��
(2)�Թܢ�������Һ������˵����__________��
(3)�Թܢ�����Һ��ɫ˵����__________��
(4)�ܷ����Թܢ��е�Һ����ȡ��ˮ�е��壬������______________________________________��
(5)д����ʮ���ѻ��õ������ϩ�Ļ�ѧ����ʽ__________________________________��
(6)ʯ���ѻ�����Ҫ������________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com