
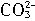 ��
��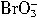 +5Br-+3CO2������
+5Br-+3CO2������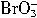 +5Br-+6H+��3Br2+3H2O��
+5Br-+6H+��3Br2+3H2O��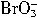 �����Դ�ǿ������˳��Ϊ��__________��
�����Դ�ǿ������˳��Ϊ��__________��  ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ѧϰ���ֱ�����ѧ�˽̰� �˽̰� ���ͣ�022
ȫ��ˮ������̲����ḻ��Լռ�������ܲ�����99���������С�����Ԫ�ء�֮�ƣ���ˮ���庬��Ϊ65 mg��L��1���乤ҵ��ȡ�����У����������������շ����÷����ǽ�����ͨ�뵽���������ӵĺ�ˮ�У�ʹ���û����������ÿ������崵�����ô�����Һ���գ�����������ữ�����ɵõ��嵥�ʣ��÷����漰���ķ�Ӧ�У���________(д�����ӷ���ʽ)����Br2��![]()
![]()
![]() ��5Br����3CO2������
��5Br����3CO2������![]() ��5Br����6H+
��5Br����6H+![]() 3Br2��3H2O�����з�Ӧ���е���������________����ԭ����________��
3Br2��3H2O�����з�Ӧ���е���������________����ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȫ��ˮ������̲����ḻ��Լռ�������ܲ�����99���������С�����Ԫ�ء�֮�ƣ���ˮ���庬��Ϊ65 mg��L-1���乤ҵ��ȡ�����У����������������շ����÷����ǽ�����ͨ�뵽���������ӵĺ�ˮ�У�ʹ���û����������ÿ������崵�����ô�����Һ���գ�����������ữ�����ɵõ��嵥�ʡ��÷����漰���ķ�Ӧ�У���__________________��д�����ӷ���ʽ������Br2+![]() ====
====![]() +5Br-+3CO2��;��
+5Br-+3CO2��;��![]() + 5Br-+6H+====3Br2+3H2O�����з�Ӧ���е���������_________________����ԭ����_________________��
+ 5Br-+6H+====3Br2+3H2O�����з�Ӧ���е���������_________________����ԭ����_________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com