
����3���������A�� CH2=CH-CH3 B�� C��CH3CH2OH
C��CH3CH2OH
��1��д��������A��C�еĹ����ŵ����� �� ��
��2��A�ڴ������������¾ۺ����ɾۺ���ķ�Ӧ����ʽΪ ����Ӧ����Ϊ ��
��3��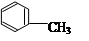 ��Ũ���������£���Ũ���Ṳ����100�淴Ӧ�Ļ�ѧ����ʽΪ�� ��
��Ũ���������£���Ũ���Ṳ����100�淴Ӧ�Ļ�ѧ����ʽΪ�� ��
��4��B���Ա����Ը�����������ɱ����ᣬд����������C������Ũ���Ṳ�ȷ���������Ӧ�Ļ�ѧ����ʽ�� ��
 �߽�������ϵ�д�
�߽�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ܺ�����άϵ���չ����Ҫǰ��֮һ��Ŀǰ����ʹ�õ���Ҫ�ܻ��ǻ�ʯȼ�ϡ���ش��������⣺
(1)��ʯȼ�ϰ���ú��ʯ�͡� (д������һ�ֻ�ʯȼ�ϵ�����)��ú̿���ɹ����У�����ȡ�Ĵ�ʩ��������������˹��ը�¹ʣ����������Ʋ���ʧ���û�ѧ����ʽ������ú����ƵƵ������˹��ը��ԭ��
��
(2)ú���������Ի���ʯ��Σ������Ŀǰú��������Ҫ��ú�е�̼��ˮ�����ķ�Ӧ��C��H2O(g)===CO��H2���÷�Ӧ��һ�����ȷ�Ӧ����Ӧ���������һ�����ɼ�Ъ���е�̼��ȼ��(�����ÿ�������)�ṩ�ģ�C��O2===CO2��
��������Ϊ������һ����Ӧ��Ҫ���ȣ�����ú�������������Ƕȿ����ò���ʧ������������ֹ۵�Ĵ������ڣ�
��
��ú����������Ϊ��ҵ�ϳɰ��ṩԭ������������ �������� �������Բ��� ���յõ������еĵ�����
(3)�ҹ�ú̿�����ʯ�ͺ���Ȼ���ʷḻ��ú������һ�����ʱ��ϡ�úҺ������Ҫ�����Ǽ��Һ���������Ƚ�ú����ΪH2��CO��CH4��Ȼ��ͨ����������̬����ת��ΪҺ̬��ͨ����ӷ��Ʊ���ȼ���к��б��������Ͳ�����ϩ������������д�ú���ϳ�ϩ���������Ļ�ѧ����ʽ��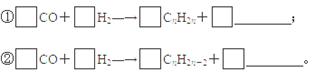
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ij����X������H2��CO��CH4�е�һ�ֻ�����ɡ���X����ȼ�գ���ȼ�պ����ɵ�����ͨ��A��B����ϴ��ƿ��
�Իش��������⣺
(1)��Aϴ��ƿ���������ӣ�Bϴ��ƿ���������䣬������X��________________��
(2)��Aϴ��ƿ���������䣬Bϴ��ƿ���������ӣ�������X��________________��
(3)��A��B����ϴ��ƿ�����������ӣ����Ʋ�X�����м�����ϣ���д�±���(�ж�����д�����֣��ɲ�������)
| ��� | �� | �� | �� | �� | �� | �� |
| X | | | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���㻯����A��һ�ֻ�������ԭ�ϣ����Դ�ú��ʯ���еõ���A��B��C��D��E��ת����ϵ������ʾ��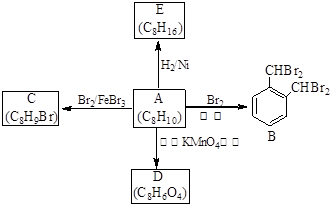
�ش��������⣺
��1��A�Ļ�ѧ������ ��E���� �������������������������
��2��A��B �ķ�Ӧ������ ���ڸ÷�Ӧ�ĸ������У���B��Ϊͬ���칹��ĸ�����Ľṹ��ʽΪ ��
��3��A��C�Ļ�ѧ����ʽΪ ��
��4��A������KMnO4��Һ��Ӧ�ɵõ�D��д��D�Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ױ��Ƕ�ú���ۺ����õõ��IJ���֮һ����ṹ��ʽΪ 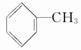 ���Իش��������⣺
���Իش��������⣺
(1)����ױ������ϵΪ_________��
| A��ͬ���칹�� | B��ͬλ�� |
| C��ͬ�������� | D��ͬϵ�� |
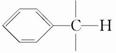 �ṹ�����ʿɱ����Ը���������������ֱ��ͼױ��ķ�����________��
�ṹ�����ʿɱ����Ը���������������ֱ��ͼױ��ķ�����________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��10�֣���ϩ��һ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ�����������ֻ�����Ʒ�ķ�Ӧ���£����ַ�Ӧ��������ȥ����
��ش��������⣺
��1��A�Ļ�ѧ������ ��
��2��B��A��Ӧ����C�Ļ�ѧ����ʽΪ ���÷�Ӧ������Ϊ ��
��3��DΪ��״�������ṹ��ʽΪ ��
��4��F�Ľṹ��ʽΪ ��
��5��D��ͬ���칹��Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ü�������������ȡ����Ӧ��ȡ����Ʒ����������ڹ�ҵ���ѳ�Ϊ��ʵ��ij��ѧ��ȤС��ͨ����ʵ������ģ���������̣�����Ƶ�ģ��װ�����£�
�������Ҫ��ش�
(1)Bװ�������ֹ��ܣ��ٿ��������ٶȣ��ھ��Ȼ�����壻��________��
(2)��V(Cl2)/V(CH4)��x��������������������Ȼ��⣬��xֵӦ________��
(3)Dװ�õ�ʯ���о��Ȼ���KI��ĩ����������_____________________________________��
(4)Eװ�õ�������___________________(����)��
| A���ռ����� | B���������� | C����ֹ���� | D�������Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
Ϊ��������β���Դ�����ɵ���Ⱦ��Ŀǰ�г����Ƴ���ʹ���Ҵ����ͣ��������м��������Ҵ��������������������������
| A���Ҵ�������һ�ֻ����� | B������ʹ���Ҵ����Ϳ��Լ����к�������ŷ� |
| C���Ҵ����ȼ������CO2��H2O | D������ʳ���Ϳ��Ƶ��Ҵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�ڱ�״����,ij�����ܶ���1.25 g��L-1,һ������ĸ�����ȫȼ������4.48 L CO2��3.6 gˮ,������ķ���ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com