
ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1�����Ǿ�����ȼ���Ҵ�����������ȷ���Ҵ��к���C��H����Ԫ�ء���Ҫ˵�����ǵľ����������֤��������Ԫ�صIJ�����________________________________________________________ _
________________________________________________________________________________________��
��֤������̼Ԫ�صIJ�����________________________________________________
_______________________________________________________________________
��2��Ҫ��ȼ�շ�������֤ʵ�Ҵ��л�������Ԫ��ʱ����ȡ��һЩʵ�����ݣ���Щ����Ӧ����________________________��
��3��Ϊȷ���Ҵ��ķ���ʽ������2���������⣬���費��Ҫ�ⶨ�Ҵ�����Է�������?
_______________________________________________________________________
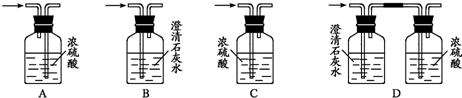
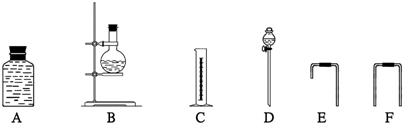
��4��Ϊ�ⶨ�Ҵ����ӽṹ������������ˮ�Ҵ��ͽ����Ʒ�Ӧ�ռ����������ķ�����ѡ��������ͼ��ʾ������(�е���������˫����Ƥ��)��

��װ�õ�����˳����______��______��______��______��_______��_______��
����֪��ˮ�ƾ����ܶ�Ϊ0��789 g��cmһ3����ȡ2��0mL�ƾ�����Ӧ��ȫ��(�ƹ���)���ռ�390 mL���塣���Ҵ��������ܱ���ȡ��������ԭ����Ϊ_______���ɴ˿�ȷ���Ҵ��ĽṹΪ______________________������______________��
��5��ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����(��д���)��______________
A����ʵ���������½���
B����ˮ�ƾ��л������״�
C����ˮ�ƾ����Ʒ�Ӧ������ȫ
��1������һ�����С�ձ��������Ҵ�ȼ�յĻ�����Ϸ����ձ��ڱ���ˮ������
�ڽ��ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ�����Ϸ���ʯ��ˮ�����
��2���Ҵ���������̼��ˮ�������ʵ����� ��3������Ҫ
��4���� D B E A F C �� 1��CH3CH2OH��CH3��O��CH3
��5��AB
��������
�����������1�����л����е�HԪ��ȼ��ʱ����H2O��������һ�����С�ձ��������Ҵ�ȼ�յĻ�����Ϸ����ձ��ڱ���ˮ�����ɿ�֤�����ʺ���Ԫ�ء�
���л����е�CԪ��ȼ��ʱ����CO2�����Խ��ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ�����Ϸ���ʯ��ˮ����ǣ���֤�����ʺ�̼Ԫ�ء�
��2�����ݶ�����̼��ˮ�������������C��HԪ�ص����������Ҵ��������Աȣ������жϳ��Ҵ�������̼Ԫ�ء�
��3�������Ҵ���������̼��ˮ�������ʵ����������C��H��Oԭ�ӵĸ�����Ϊ��2:6:1������C��H��O�ijɼ������֪���Ҵ��ķ���ʽֻ��ΪC2H6O�����Բ���Ҫ�ⶨ�Ҵ�����Է�������������ȷ���Ҵ��ķ���ʽ��
��4���ٷ�Һ©������ƿ����Ϊ��Ӧװ�ã�����ͨ��E�̵��ܽ���A��A�е�ˮ��F������Ͳ�����������������롢˳��Ϊ�� D B E A F C
���Ҵ������ʵ���Ϊ��2.0mL��0.789g•cm-3��46g/mol=0.034mol�����ɵ�����Ϊ��0.39L��22.4L/mol=0.017mol������ �Ҵ��������ܱ���ȡ��������ԭ����Ϊ1����ȷ���Ҵ��ĽṹΪCH3CH2OH��������CH3��O��CH3
��5��A����ʵ���������½��У�����Ħ���������22.4L/mol,��ɽ��ƫ�ߣ���ȷ��B����Ϊ��ͬ�����ļ״���Ӧ���ɵ��������࣬��ˮ�ƾ��л������״�����ɽ��ƫ�ߣ���ȷ��C����ˮ�ƾ����Ʒ�Ӧ������ȫ�����ɵ�����ƫ�٣�����ƫ�ͣ�����
���㣺���⿼�黯ѧʵ��Ļ����������Ӻͻ���������ʵ�鷽���ķ�������������ʵ�����ݴ����ͼ��㡣


 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| �� |
| ||
| �� |
| ����ø |
| �ƻ�ø |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ��ѧ������ȤС���ͬѧ���ⶨijƷ��ζ����ʳ�εĺ����������Ǹ�С�������е�ʵ�鲽�裺
�ٳ�ȡijƷ�ƴ�װζ����Ʒ10.0g�����ձ��У�������������ˮ�ܽ⣻
�� ��
�� ��
��������ˮϴ�ӳ���2��3�Σ�
�ݽ�������ɡ���������ù�������4.90g��
��������ʵ�鲽��ش��������⣺
��1�����㲹����ȱ��ʵ�鲽�裺�� ��
��2��ʵ������õIJ��������� ��
��3����������Ƿ�ϴ���ķ����� ��
��4����ζ���̱��ϱ�ע�����Ȱ����ƺ�����80.0%��NaCl������20.0%���������Ʒ�Ƿ�ϸ���ϸ������ϸ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
( 16�� ) ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ�����: �Ҵ������Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
( 1 ) ���Ǿ�����ȼ���Ҵ�����������ȷ���Ҵ��к���C��H����Ԫ�ء���Ҫ˵�����ǵľ������: ��֤��������Ԫ�صIJ����� _________________ ����֤������̼Ԫ�صIJ����� ____________________ ��
( 2 ) Ҫ��ȼ�շ�������֤ʵ�Ҵ��л�������Ԫ��ʱ����ȡ��һЩʵ�����ݣ���Щ����Ӧ���� __________________ ��
( 3 ) Ϊȷ���Ҵ��ķ���ʽ���� ( 2 ) �������⣬���費��ⶨ�Ҵ�����Է���������
( 4 ) Ϊ�ⶨ�Ҵ����ӽṹ������������ˮ�Ҵ��ͽ����Ʒ�Ӧ�ռ����������ķ�����ѡ��������ͼ��ʾ������ ( �е���������˫����Ƥ�� ) ��

��װ�õ�����˳���� ________ �� _________ �� _________ �� _________ ��
__________ �� ______________ ��
����ʵ��֤���Ҵ��ķ��ӽṹ��CH3CH2OH������CH3OCH3�������� _________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
( 16�� ) ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ�����: �Ҵ������Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
( 1 ) ���Ǿ�����ȼ���Ҵ�����������ȷ���Ҵ��к���C��H����Ԫ�ء���Ҫ˵�����ǵľ������:��֤��������Ԫ�صIJ����� _________________ ����֤������̼Ԫ�صIJ����� ____________________ ��
( 2 ) Ҫ��ȼ�շ�������֤ʵ�Ҵ��л�������Ԫ��ʱ����ȡ��һЩʵ�����ݣ���Щ����Ӧ���� __________________ ��
( 3 ) Ϊȷ���Ҵ��ķ���ʽ���� ( 2 ) �������⣬���費��ⶨ�Ҵ�����Է���������
( 4 ) Ϊ�ⶨ�Ҵ����ӽṹ������������ˮ�Ҵ��ͽ����Ʒ�Ӧ�ռ����������ķ�����ѡ��������ͼ��ʾ������ ( �е���������˫����Ƥ�� ) ��
��װ�õ�����˳����________ �� _________ ��_________ �� _________ ��
__________ �� ______________ ��
����ʵ��֤���Ҵ��ķ��ӽṹ��CH3CH2OH������CH3OCH3�������� _________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com