

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
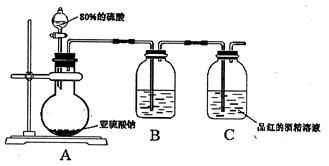
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��NaNO3��Һ | B��CCl4 | C���� | D��ϡ���� |
�鿴�𰸺ͽ���>>
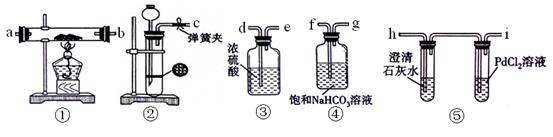
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
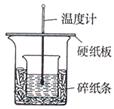
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
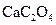 Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����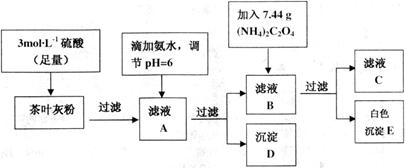
| ���� | 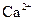 | 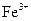 |
| ��ȫ����ʱ��pH | 13 | 4.1 |
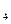 ����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
����Һ�ζ�C��Һʱ�������ķ�ӦΪ��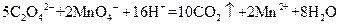 ��
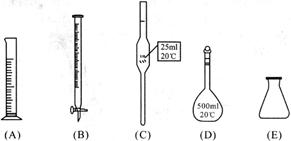
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
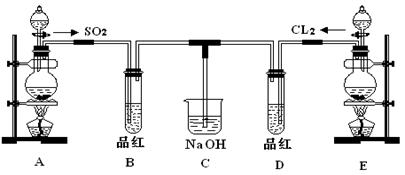
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���۲� | B��ʵ �� �� | C������ | D���Ƚ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
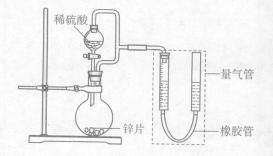
 ��
��| ��� | V��H2SO4��/mL | C(H2SO4)/mol��L-1 | t/s |
| I | 40 | 1 | t1 |
| II | 40 | 4 | t2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
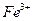 ����ܺ���
����ܺ���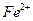 ����Ҫȷ�����е�
����Ҫȷ�����е�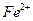 ��Ӧѡ��
��Ӧѡ��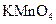 ��Һ
��Һ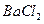 ��Һ�����ʵ�������ø������2.33g���ɴ���֪����Y��
��Һ�����ʵ�������ø������2.33g���ɴ���֪����Y��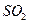 ���������Ϊ ��
���������Ϊ ��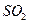 ��������Ľ����ͬѧ����Ϊ����Y�л����ܺ������������岢�����²��룺
��������Ľ����ͬѧ����Ϊ����Y�л����ܺ������������岢�����²��룺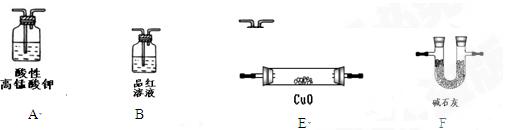
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ƽ��ȡ4.0gNaOH���壬��100mL��Ͳ����1.00mol/L��NaOH��Һ |
| B����25mL�ĵζ��ܣ���װ�б�NaOH��Һ���ζ�δ֪Ũ�ȵ�������ȥNaOH��Һ22.32mL |
| C����pH��ֽ���������ˮ��pHΪ4 |
| D�������£��������ռ�500mLNO2���壬���NO2��������ʵ���Ϊ��0.5/22.4��mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com