
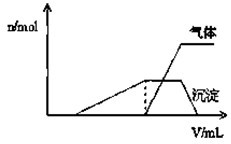
 1Lij�����Һ�����ܺ��е��������±���
1Lij�����Һ�����ܺ��е��������±���| ���ܴ������е������� | H+NH4+Al3+K+ |
| ���ܴ������е������� | Cl-Br-I?ClO?AlO2- |
| Cl2���������״���� | 2.8L | 5.6L | 11.2L |
| n��Cl-�� | 1.25mol | 1.5mol | 2mol |
| n��Br-�� | 1.5mol | 1.4mol | 0.9mol |
| n��I-�� | a mol | 0 | 0 |
| 2.8 |
| 22.4 |
| 2.8 |
| 22.4 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ʱ����Na2O2��H2O2����Ӧ���ѡ����ͬ�����巢��װ�� |
| B��������ʱ���ñ���NaHCO3��Һ��Ũ���Ά������ |
| C������ϩʱ������ˮ���������ſ������ռ����� |
| D���ƶ�������ʱ����ˮ��NaOH��Һ����β�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
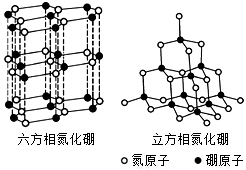 ������BN�������ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ����в�״�ṹ�������������������൪�����dz�Ӳ���ϣ����������ĥ�ԣ����ǵľ���ṹ��ͼ��ʾ��
������BN�������ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ����в�״�ṹ�������������������൪�����dz�Ӳ���ϣ����������ĥ�ԣ����ǵľ���ṹ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
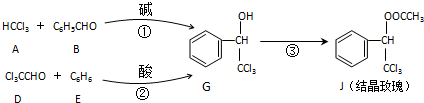
| KOH |
 +
+
| �� |
 +H2O����Ӧ·�ߢڵõ�һ�ָ������˴Ź���������4��壬�������շ�����֮��Ϊ
+H2O����Ӧ·�ߢڵõ�һ�ָ������˴Ź���������4��壬�������շ�����֮��Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
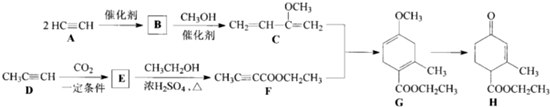
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
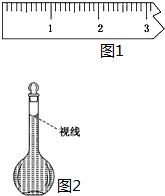 ʵ������Ҫ����0.50mol/L NaCl��Һ480mL�������в������������ʵ������֣���ʹ��������������
ʵ������Ҫ����0.50mol/L NaCl��Һ480mL�������в������������ʵ������֣���ʹ���������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� ���� |
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | |||
| �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ��û��CO2���϶���H2 |
| B��һ����CO��CO2��ˮ����? |
| C��һ����H2��CO2��HCl |
| D��������CO2��NH3��ˮ����? |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com