
 �������ϵ�д�
�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
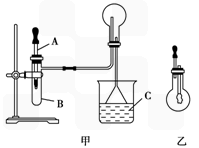
������ͼ������ijѧУ����ʦ�Ʊ�NH3����������ʵ��ʱ�ĸĽ�װ�á���ͼ�װ�������װ�ã���ȡ2g�����Ȼ��װ���Թܵײ����ٿ��ٳ�ȡ2g�������Ƹ������Ȼ���Ϸ��������ô��еιܵ������������ι�Ԥ������Լ2mLŨ��ˮ�����ձ���ʢ���з�̪��Һ��ˮ����Ũ��ˮ�����Թ���������۲쵽�Թ��ڷ������ҷ�Ӧ���д������ݡ�
������������NH3��Բ����ƿȡ�£���װ��ͼ����ʾ��װ�ã���ͷ�ι�������Ԥ����2mLH2O����ʱС����ϵ�ڲ������ϳ���Ȼ�ɳ�״̬�����ι��ڵ�ˮ����������ƿ�У�����ζ���ƿ��ͨ���۲�ʵ������������֤NH3��ij�����ʡ���Ҫ��ش��������⣺
��1����ѧ��ѧ�̲�����������O2��ͬ���Ʊ�װ����������ȡNH3�ģ��û�ѧ����ʽΪ______________________________________________________________��
��2��������ijͬѧ��������ʦ����ͼ����ȡNH3��ԭ��������е�������_____________
����NH3?H2O����ƽ��NH3��H2O![]() NH3?H 2O
NH3?H 2O![]() NH4����OH������NaOHʹƽ�������ƶ�
NH4����OH������NaOHʹƽ�������ƶ�
����NH3?H2O����ƽ��NH3��H2O![]() NH3?H2O
NH3?H2O![]() NH4����OH������NH4Clʹƽ�������ƶ�
NH4����OH������NH4Clʹƽ�������ƶ�
��NaOH����ˮʱ���ȣ�ʹ��ϵ���¶����ߣ�NH3���ܽ�ȼ�С�����в���NH3�ݳ�
��NH4Cl��NaOH�ڴ�����¿ɷ�Ӧ����NH3����NH4����OH��![]() NH3����H2O
NH3����H2O
��NH4Cl��ֽ��ͷų�NH3
��3��ͼ���е�NH4Cl��NaOH���������ܷ���CaO������棿___����ܡ����ܡ�����
��4������ж�ͼ������ƿ������NH3��________________________________________��
��5��ͼ���н�ͷ�ι��е�ˮ������ƿ�У��۲쵽��������_________����˵����NH3__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
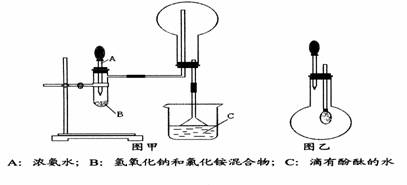
(I) ��ͼ������ijѧУ����ʦ�Ʊ�NH3 ����������ʵ��ʱ�ĸĽ�װ�á���ͼ�װ�������װ�ã���ȡ2g �����Ȼ��װ���Թܵײ����ٿ��ٳ�ȡ2g �������Ƹ������Ȼ���Ϸ��������ô��еιܵ������������ι�Ԥ������Լ2mL Ũ��ˮ�����ձ���ʢ���з�̪��Һ��ˮ����Ũ��ˮ�����Թ���������۲쵽�Թ��ڷ������ҷ�Ӧ���д�������.
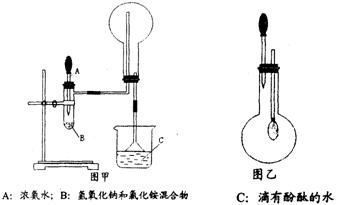
������������NH3 ��Բ����ƿȡ�£���װ��ͼ����ʾ��װ�ã���ͷ�ι�������Ԥ����2mLH2O ����ʱС����ϵ�ڲ������ϳ���Ȼ�ɳ�״̬�����ι��ڵ�ˮ����������ƿ�У�����ζ���ƿ��ͨ���۲�ʵ������������֤NH3 ��ij�����ʡ���Ҫ��ش��������⣺
(1)��ѧ��ѧ�̲�����������O2��ͬ���Ʊ�װ����������ȡNH3�ģ��û�ѧ����ʽΪ:__________________________________________________________
(2)������ijͬѧ��������ʦ����ͼ����ȡNH3��ԭ��������е�������__________��
����NH3 �� H2O ����ƽ��![]() ʹƽ�������ƶ�
ʹƽ�������ƶ�
����NH3�� H2O ����ƽ��![]() ʹƽ�������ƶ�
ʹƽ�������ƶ�
��Na0H ����ˮʱ���ȣ�ʹ��ϵ���¶����ߣ�NH3 ���ܽ�ȼ�С
��NH4Cl��NaOH �ڴ�����¿ɷ�Ӧ����NH3��![]()
��NH4Cl ��ֽ��ͷų�NH3
(3)ͼ���е�NH4Cl ��NaOH ���������ܷ���CaO ������� (��ܡ��롱���ܡ�)
(4)����ж�ͼ������ƿ������NH3 ?_____________________________________________
(5)ͼ���н�ͷ�ι��е�ˮ������ƿ�۲쵽�������� ��˵����NH3

(��)����ͼ��ʾ����B����װ��500 mLˮ���ݻ�Ϊa mL���Թ�A������NO2��NO�Ļ�����壨��״���������Թ�A������B�۵�ˮ�С���ַ�Ӧ���Թ�A��������������Ϊ0.5a mL����ԭ���������NO2��NO�����ʵ���֮��Ϊ
ͨ��������C������0.5a mL������Թ�A�г���ͨ��������A�п��ܹ۲쵽�������ǣ�
___________________________________________________
�йط�Ӧ�Ļ�ѧ����ʽΪ��___________________________________________
���Թ�A�г�������ʱֹͣͨ��������Ȼ���Թ�ȡ��ˮ�ۣ���ͨ�����������Ϊ ________mL��ˮ��B����Һ�����ʵ���Ũ��Ϊ mol��L-1(����Һ�������Ϊ500 mL)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com