
 ______�����������С�����䡱����
______�����������С�����䡱����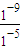 =10-4��
=10-4��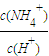 ����Ũ�ȱ�ֵ��С��
����Ũ�ȱ�ֵ��С��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(NH4+) | c(H+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ����һ�и�����ͷ���Ի�ѧ�Ծ����������� ���ͣ������
��֪�����µ������£�pH��Ϊ5��H2SO4��Һ��NH4Cl��Һ���ش��������⣺
��1����ȡ5mL������Һ���ֱ��ˮϡ����50mL��pH�ϴ����________��Һ
��2����ȡ5mL������Һ���ֱ���ȣ��¶���ͬ����pH��С����________��Һ
��3��H2SO4��Һ��NH4Cl��Һ����ˮ�������c(H+)֮��Ϊ__________
��4��ȡ5mL NH4Cl��Һ����ˮϡ����50mL��c(H+) __________ 10-6mol��L-1���>������<����=������
c(NH4+)��c(H+)_______________�����������С�����䡱��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ������ͷ���Ի�ѧ�Ծ��������棩 ���ͣ������
��֪�����µ������£�pH��Ϊ5��H2SO4��Һ��NH4Cl��Һ���ش��������⣺
��1����ȡ5mL������Һ���ֱ��ˮϡ����50mL��pH�ϴ����________��Һ
��2����ȡ5mL������Һ���ֱ���ȣ��¶���ͬ����pH��С����________��Һ
��3��H2SO4��Һ��NH4Cl��Һ����ˮ�������c(H+)֮��Ϊ__________
��4��ȡ5mL NH4Cl��Һ����ˮϡ����50mL��c(H+) __________ 10-6mol��L-1���>������<����=������
c(NH4+)��c(H+)_______________�����������С�����䡱��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����µ������£�pH��Ϊ5��H2SO4��Һ��NH4Cl��Һ���ش��������⣺
��1������Һ��c(H+) ?? c(OH-)=___________
��2����ȡ5mL������Һ���ֱ��ˮϡ����50mL��pH�ϴ����________��Һ
��3����ȡ5mL������Һ���ֱ���ȵ�90�棬pH��С����________��Һ
��4������Һ����ˮ�������c(H+)�ֱ�Ϊ��H2SO4��Һ________��NH4Cl��Һ__________
��5��ȡ5mL NH4Cl��Һ����ˮϡ����50mL��c(H+) 10-6mol��L-1���>������<����=������c(NH4+)��c(H+) �����������С�����䡱��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com