
¢Ł³ĘȔѳʷ0.100 0 g£¬¼ÓĖįČܽā£¬µĆµ½ŗ¬Pb2+µÄČÜŅŗ”£
¢ŚŌŚ¼ÓČČĢõ¼žĻĀÓĆ¹żĮæK2Cr2O7½«Pb2+³ĮµķĪŖPbCrO4£¬ĄäČ“ŗó¹żĀĖĻ“µÓ³Įµķ”£
¢Ū½«PbCrO4³ĮµķÓĆĖįČÜŅŗČܽā(³ĮµķČܽāµÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ2PbCrO4+2H+![]() 2Pb2++
2Pb2++![]() +H2O)£¬¼ÓČė¹żĮæKI£¬ŌŁÓĆ0.100 0 mol”¤L-1 Na2S2O3ČÜŅŗµĪ¶Ø£¬µ½µĪ¶ØÖÕµćŹ±ÓĆČ„12.00 mL(µĪ¶Ø¹ż³ĢÖŠĄė×Ó·½³ĢŹ½ĪŖ£ŗI2+2
+H2O)£¬¼ÓČė¹żĮæKI£¬ŌŁÓĆ0.100 0 mol”¤L-1 Na2S2O3ČÜŅŗµĪ¶Ø£¬µ½µĪ¶ØÖÕµćŹ±ÓĆČ„12.00 mL(µĪ¶Ø¹ż³ĢÖŠĄė×Ó·½³ĢŹ½ĪŖ£ŗI2+2![]()
![]() 2I-+
2I-+![]() )”£Ōņ£ŗ
)”£Ōņ£ŗ
(1)Š“³ö²½Öč¢ŪÖŠ¼ÓČė¹żĮæKIŗóČÜŅŗÖŠ·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½______________________”£
(2)ÓƱź×¼ČÜŅŗµĪ¶ØŹ±ĖłÓƵÄÖøŹ¾¼ĮŹĒ___________”£(Š“ŹŌ¼ĮĆū³Ę)
(3)¼ĘĖćŹŌŃłÖŠPb3O4µÄÖŹĮæ·ÖŹż”£(PbµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ207.2)
(1)![]() +6I-+14H+
+6I-+14H+![]() 2Cr3++3I2+7H2O
2Cr3++3I2+7H2O
(2)µķ·Ū
(3)Pb3O4”Ŗ3Pb2+”Ŗ3/2Cr2O72-”Ŗ3”Į3/2I2”Ŗ3”Į3/2”Į2![]()
685.6 9
x g 0.100 0 mol”¤L-1”Į12.00 mL”Į10
x=0.091
½āĪö£ŗ±¾ĢāŹĒĄūÓĆŃõ»Æ»¹Ō·“Ó¦ŌĄķ½ųŠŠµÄµĪ¶Ø²ā¶Ø£¬2 mol PbCr2O4×ŖĪŖ1 mol ![]() £¬
£¬ ![]() °ŃI-Ńõ»ÆĪŖI2£¬I2°Ń
°ŃI-Ńõ»ÆĪŖI2£¬I2°Ń![]() Ńõ»ÆĪŖ
Ńõ»ÆĪŖ![]() ”£ŅņĪŖI2Ź¹µķ·Ū±äĄ¶£¬ÖøŹ¾¼ĮĪŖµķ·ŪČÜŅŗ£¬µĪ¶ØÖĮĄ¶É«ĶŹČ„“ļµĪ¶ØÖÕµć”£¼ĘĖćPb3O4µÄÖŹĮæ·ÖŹżæÉøł¾ŻĻĀĮŠ¹ŲĻµŹ½ĶĘĖć”£
”£ŅņĪŖI2Ź¹µķ·Ū±äĄ¶£¬ÖøŹ¾¼ĮĪŖµķ·ŪČÜŅŗ£¬µĪ¶ØÖĮĄ¶É«ĶŹČ„“ļµĪ¶ØÖÕµć”£¼ĘĖćPb3O4µÄÖŹĮæ·ÖŹżæÉøł¾ŻĻĀĮŠ¹ŲĻµŹ½ĶĘĖć”£
Pb3O4”Ŗ![]()
![]() ”Ŗ
”Ŗ![]() I2”Ŗ9
I2”Ŗ9![]()
685.6 9
x 0.100 mol”¤L-1”Į
x=0.091
w(Pb3O4)=![]() ”Į100%=91.4%”£
”Į100%=91.4%”£


 ѧ¶ųÓÅĻĪ½Ó½Ģ²ÄÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
ѧ¶ųÓÅĻĪ½Ó½Ģ²ÄÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø Š”ѧæĪĢĆ×÷ŅµĻµĮŠ“š°ø
Š”ѧæĪĢĆ×÷ŅµĻµĮŠ“š°ø ½š²©ŹæŅ»µćČ«ĶØĻµĮŠ“š°ø
½š²©ŹæŅ»µćČ«ĶØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
ŌŚ21”ęŗĶ³ä·Ö½Į°čĻĀ£¬½«²»Ķ¬Ģå»ż1.0 mol”¤L”Ŗ1 HClČÜŅŗŗĶĪ“ÖŖÅØ¶ČµÄNaOHČÜŅŗ»ģŗĻ¾łŌČŗó²āĮæ²¢¼ĒĀ¼ČÜŅŗĪĀ¶Č£¬ŹµŃé½į¹ūČēĻĀ£ŗ
| ŃĪĖįµÄĢå»żV£ØmL£© | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 | 45.0 |
| NaOHµÄĢå»ż£ØmL) | 45.0 | 40.0 | 35.0 | 30.0 | 25.0 | 20.0 | 15.0 | 10.0 | 5.0 |
| ČÜŅŗĪĀ¶Čt£Ø”ę£© | 22.2 | 23.3 | 24.6 | 25.8 | 27.0 | 27.8 | 26.1 | 24.4 | 22.8 |
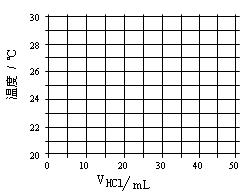 £Ø1£©ŌŚøų¶ØµÄ×ų±źĶ¼ÉĻ»ę³öČÜŅŗĪĀ¶ČÓėŃĪĖįĢå»żµÄ¹ŲĻµĶ¼”£
£Ø1£©ŌŚøų¶ØµÄ×ų±źĶ¼ÉĻ»ę³öČÜŅŗĪĀ¶ČÓėŃĪĖįĢå»żµÄ¹ŲĻµĶ¼”£
£Ø2£©¼Ł¶ØĖį¼īĒ”ŗĆĶźČ«·“Ó¦Ē°ŗó£¬ČÜŅŗĪĀ¶ČÓėŃĪĖįĢå»żæÉŅŌ½üĖĘ
µŲČĻĪŖ³ŹĻߊŌ¹ŲĻµ”£ĒėŠ“³öČÜŅŗĪĀ¶ČtÓėŃĪĖįĢå»żVµÄĻߊŌ
¹ŲĻµŹ½£ØĒėÓĆŗ¬ÓŠtŗĶVµÄŹ½×Ó±ķŹ¾£© ”¢ ”£
£Ø3£©ĖłÓĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČ= ”£
±øÓĆ . ĖÄŃõ»ÆČżĒ¦Ė×Ćū”°Ē¦µ¤”±»ņ”°ŗģµ¤”±£¬ÓÉÓŚÓŠŃõ»ÆŠŌ±»“óĮæµŲÓĆÓŚÓĶĘį“¬²°ŗĶĒÅĮŗøּܷĄŠā£¬Ęä»ÆѧŹ½æÉŠ“ĪŖ2PbO”¤PbO2”£Óū²ā¶ØÄ³ŃłĘ·ÖŠĖÄŃõ»ÆČżĒ¦ŗ¬Į棬½ųŠŠČēĻĀ²Ł×÷£ŗ
¢Ł³ĘȔѳʷ0.1000g£¬¼ÓĖįČܽā£¬µĆµ½ŗ¬Pb2£«µÄČÜŅŗ”£
¢ŚŌŚ¼ÓČČĢõ¼žĻĀÓĆ¹żĮæK2Cr2O7½«Pb2£«³ĮµķĪŖPbCrO4£¬ĄäČ“ŗó¹żĀĖĻ“µÓ³Įµķ”£
¢Ū½«PbCrO4³ĮµķÓĆĖįČÜŅŗČܽā£Ø³ĮµķČܽāµÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ2PbCrO4+2H£«£½
2Pb2+Cr2O72£+H2O£©£¬¼ÓČė¹żĮæKI£¬ŌŁÓĆ0.1000mol”¤LØD1 Na2S2O3ČÜŅŗµĪ¶Ø£¬µ½µĪ¶ØÖÕµćŹ±ÓĆ
Č„12.00mL£ØµĪ¶Ø¹ż³ĢÖŠĄė×Ó·½³ĢŹ½ĪŖ£ŗI2£«2S2O32££½2I££«S4O62££©”£
Ōņ£ŗ(1)Š“³ö²½Öč¢ŪÖŠ¼ÓČė¹żĮæKIŗóČÜŅŗÖŠ·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½ ”£
£Ø2£©ÓƱź×¼ČÜŅŗµĪ¶ØŹ±ĖłÓƵÄÖøŹ¾¼ĮŹĒ ”££ØŠ“ŹŌ¼ĮĆū³Ę£©
£Ø3£©¼ĘĖćŹŌŃłÖŠPb3O4µÄÖŹĮæ·ÖŹż”£(PbµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ207.2)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010”Ŗ2011ѧğŗŚĮś½Ź”“óĒģŹµŃé֊ѧø߶žĻĀѧʌæŖѧ²āŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø9·Ö£©ĖÄŃõ»ÆČżĒ¦Ė×Ćū”°Ē¦µ¤”±»ņ”°ŗģµ¤”±£¬ÓÉÓŚÓŠŃõ»ÆŠŌ±»“óĮæµŲÓĆÓŚÓĶĘį“¬²°ŗĶĒÅĮŗøּܷĄŠā£¬Ęä»ÆѧŹ½æÉŠ“ĪŖŃõ»ÆĪļ2PbO”¤PbO2»ņŃĪPb2PbO4”£Óū²ā¶ØÄ³ŃłĘ·ÖŠĖÄŃõ»ÆČżĒ¦ŗ¬Į棬½ųŠŠČē ĻĀ²Ł×÷£ŗ
ĻĀ²Ł×÷£ŗ
¢Ł³ĘȔѳʷ0.1000 g£¬¼Ó»¹ŌŠŌĖįČܽā£¬µĆµ½ŗ¬Pb2£«µÄČÜŅŗ”£
¢ŚŌŚ¼ÓČČĢõ¼žĻĀÓĆ¹żĮæK2Cr2O7½«Pb2£«³ĮµķĪŖPbCrO4£¬ĄäČ“ŗó¹żĀĖĻ“µÓ³Įµķ”£
¢Ū½«PbCrO4³ĮµķÓĆĖįČÜŅŗČܽā£ØĄė×Ó·½³ĢŹ½ĪŖ2PbCrO4£«2H£«£½2Pb2£«£«Cr2O72££«H2O£©£¬¼ÓČė¹żĮæKI
ČÜŅŗ£¬ŌŁÓĆNa2S2O3±ź×¼ČÜŅŗµĪ¶Øµ½ÖÕµć£ØµĪ¶Ø¹ż³ĢÖŠĄė×Ó·½³ĢŹ½ĪŖ£ŗI2£«2S2O32££½2I££«S4 O62££©”£
O62££©”£
(1)ŅŃÖŖPbµÄŌ×ÓŠņŹżŹĒ82£¬ĒėŠ“³öPbĪ»ÓŚÖÜĘŚ±ķµÄµŚ_____ÖÜĘŚ____ __×唣
__×唣
£Ø2£©ŌŚPbCrO4×ĒŅŗÖŠ¼ÓČėÉŁĮæĖį£¬ŌņKsp(PbCrO4) £ØĢī”°Ōö“ó”±”¢ ”°¼õŠ””±”¢”°²»±ä”±£©
”°¼õŠ””±”¢”°²»±ä”±£©
£Ø3£©Š“³ö²½Öč¢ŪÖŠ¼ÓČė¹żĮæKIŗóČÜŅŗÖŠ·¢Éś·“Ó¦µÄĄė×Ó·“Ó¦·½³ĢŹ½ ”£
£Ø4£©ÓūĒóŹŌŃłÖŠPb3O4µÄÖŹĮæ·ÖŹż£¬»¹ŠčŅŖµÄŹż¾ŻÓŠ ”£
£Ø5£©Fe3O4ÓėPb3O4ĻąĖĘ£¬ĘäŃõ»ÆĪļŗĶŃĪµÄŠĪŹ½·Ö±šŹĒ ”¢ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģŗŚĮś½Ź”ø߶žĻĀѧʌæŖѧ²āŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø9·Ö£©ĖÄŃõ»ÆČżĒ¦Ė×Ćū”°Ē¦µ¤”±»ņ”°ŗģµ¤”±£¬ÓÉÓŚÓŠŃõ»ÆŠŌ±»“óĮæµŲÓĆÓŚÓĶĘį“¬²°ŗĶĒÅĮŗøּܷĄŠā£¬Ęä»ÆѧŹ½æÉŠ“ĪŖŃõ»ÆĪļ2PbO”¤PbO2»ņŃĪPb2PbO4”£Óū²ā¶ØÄ³ŃłĘ·ÖŠĖÄŃõ»ÆČżĒ¦ŗ¬Į棬½ųŠŠČēĻĀ²Ł×÷£ŗ
¢Ł³ĘȔѳʷ0.1000 g£¬¼Ó»¹ŌŠŌĖįČܽā£¬µĆµ½ŗ¬Pb2£«µÄČÜŅŗ”£
¢ŚŌŚ¼ÓČČĢõ¼žĻĀÓĆ¹żĮæK2Cr2O7½«Pb2£«³ĮµķĪŖPbCrO4£¬ĄäČ“ŗó¹żĀĖĻ“µÓ³Įµķ”£
¢Ū½«PbCrO4³ĮµķÓĆĖįČÜŅŗČܽā£ØĄė×Ó·½³ĢŹ½ĪŖ2PbCrO4£«2H£«£½2Pb2£«£«Cr2O72££«H2O£©£¬¼ÓČė¹żĮæKI
ČÜŅŗ£¬ŌŁÓĆNa2S2O3±ź×¼ČÜŅŗµĪ¶Øµ½ÖÕµć£ØµĪ¶Ø¹ż³ĢÖŠĄė×Ó·½³ĢŹ½ĪŖ£ŗI2£«2S2O32££½2I££«S4O62££©”£
(1)ŅŃÖŖPbµÄŌ×ÓŠņŹżŹĒ82£¬ĒėŠ“³öPbĪ»ÓŚÖÜĘŚ±ķµÄµŚ_____ÖÜĘŚ______×唣
£Ø2£©ŌŚPbCrO4×ĒŅŗÖŠ¼ÓČėÉŁĮæĖį£¬ŌņKsp(PbCrO4) £ØĢī”°Ōö“ó”±”¢ ”°¼õŠ””±”¢”°²»±ä”±£©
£Ø3£©Š“³ö²½Öč¢ŪÖŠ¼ÓČė¹żĮæKIŗóČÜŅŗÖŠ·¢Éś·“Ó¦µÄĄė×Ó·“Ó¦·½³ĢŹ½ ”£
£Ø4£©ÓūĒóŹŌŃłÖŠPb3O4µÄÖŹĮæ·ÖŹż£¬»¹ŠčŅŖµÄŹż¾ŻÓŠ ”£
£Ø5£©Fe3O4ÓėPb3O4ĻąĖĘ£¬ĘäŃõ»ÆĪļŗĶŃĪµÄŠĪŹ½·Ö±šŹĒ ”¢ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com