
ЁОЬтФПЁПЬњМАЦфЛЏКЯЮяЪЧШеГЃЩњЛюЩњВњжагІгУЙуЗКЕФВФСЯЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЛљЬЌЬњдзгЕФМлЕчзгЙьЕРБэДяЪНЮЊ_____________________ ЁЃ
ЃЈ2ЃЉЬњдЊЫиГЃМћЕФРызггаFe2+КЭFe3+ЃЌЮШЖЈадFe2+____Fe3+(ЬюЁАДѓгкЁБЁАЁБЛђЁАаЁгкЁБ)ЃЌдвђЪЧ______________________ ЁЃ
ЃЈ3ЃЉФЩУзбѕЛЏЬњФмДпЛЏЛ№М§ЭЦНјМСNH4ClO4ЕФЗжНтЃЌNH4+ЕФНсЙЙЪНЮЊ_________________(БъГіХфЮЛМќ)ЃЌПеМфЙЙаЭЮЊ_______________ЃЌЦфжаЕЊдзгЕФдгЛЏЗНЪНЮЊ_______________ЃЛгыClO4-ЛЅЮЊЕШЕчзгЬхЕФЗжзгЛђРызгга______(ШЮаДвЛжж)ЁЃ
ЃЈ4ЃЉФГжжРызгаЭЬњЕФбѕЛЏЮяОЇАћШчЭМЫљЪОЃЌЫќгЩAЁЂBЗНПщзщГЩЁЃдђИУбѕЛЏЮяжаFe2+ЁЂFe3+ЁЂO3-ЕФИіЪ§БШЮЊ___________(ЬюзюМђећЪ§БШ)ЁЃ
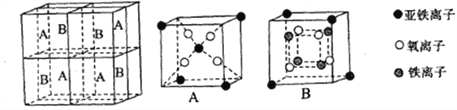
ЃЈ5ЃЉЬњгаІФЁЂІУЁЂІСШ§жжЭЌЫивьаЮЬхЃЌШчЯТЭМЫљЪОЁЃ

ІУ-FeОЇЬхЕФвЛИіОЇАћжаЫљКЌгаЕФЬњдзгЪ§ЮЊ________ЃЌІФ-FeЁЂІС-FeСНжжОЇАћжаЬњдзгЕФХфЮЛЪ§жЎБШЮЊ_________ЁЃ
вбжЊІФ-FeОЇЬхЕФУмЖШЮЊdg/cm3ЃЌNAБэЪОАЂЗќйЄЕТТоГЃЪ§ЕФЪ§жЕЃЌдђFeдзгАыОЖЮЊ_______Pm(СаБэДяЪН)ЁЃ
ЁОД№АИЁП 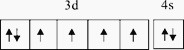 аЁгк Fe2+ЕФМлЕчзгХХВМЪНЮЊ3d6ЃЌFe3+ЕФМлЕчзгХХВМЪНЮЊ3d5ЃЌFe3+ ЕФ3dФмМЖЮЊАыТњзДЬЌНЯЮШЖЈ
аЁгк Fe2+ЕФМлЕчзгХХВМЪНЮЊ3d6ЃЌFe3+ЕФМлЕчзгХХВМЪНЮЊ3d5ЃЌFe3+ ЕФ3dФмМЖЮЊАыТњзДЬЌНЯЮШЖЈ 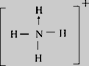 е§ЫФУцЬхаЮ sp3дгЛЏ CCl4ЁЂPO43- 1ЃК2ЃК4 4 4ЃК3
е§ЫФУцЬхаЮ sp3дгЛЏ CCl4ЁЂPO43- 1ЃК2ЃК4 4 4ЃК3 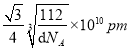
ЁОНтЮіЁПЃЈ1ЃЉЃЉFeдЊЫиЮЊ26КХдЊЫиЃЌдзгКЫЭтга26ИіЕчзгЃЌЫљвдКЫЭтЕчзгХХВМЪНЮЊЃК1s22s22p63s23p63d64s2ЃЌЛљЬЌЬњдзгЕчзгХХВМЮЊЕФМлЕчзгЙьЕРБэДяЪНЮЊ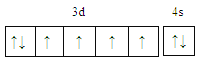 ЃЛе§ШЗД№АИЃК
ЃЛе§ШЗД№АИЃК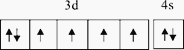 ЁЃ
ЁЃ
ЃЈ2ЃЉЬњдЊЫиГЃМћЕФРызггаFe2+КЭFe3+ЃЌЮШЖЈадFe2+аЁгкFe3+ЃЌдвђЪЧFe2+ЕФМлЕчзгХХВМЪНЮЊ3d6ЃЌFe3+ЕФМлЕчзгХХВМЪНЮЊ3d5ЃЌFe3+ЕФ3dФмМЖЮЊАыТњзДЬЌНЯЮШЖЈЃЛе§ШЗД№АИЃКаЁгк ЃЛ Fe2+ЕФМлЕчзгХХВМЪНЮЊ3d6ЃЌFe3+ЕФМлЕчзгХХВМЪНЮЊ3d5ЃЌFe3+ ЕФ3dФмМЖЮЊАыТњзДЬЌНЯЮШЖЈЁЃ
ЃЈ3ЃЉФЩУзбѕЛЏЬњФмДпЛЏЛ№М§ЭЦНјМСNH4ClO4ЕФЗжНтЃЌNH4+ЕФНсЙЙЪНЮЊ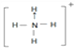 ЃЌПеМфЙЙаЭЮЊе§ЫФУцЬхаЮЃЌЦфжаЕЊдзгЕФМлВуЕчзгЖдЮЊ4ЃЌдгЛЏЗНЪНЮЊsp3дгЛЏЃЛClO4-ЪЧ5дзгЁЂ32МлЕчзгЕФРызгЃЌгыClO4-ЛЅЮЊЕШЕчзгЬхЕФЗжзгЛђРызггаCCl4ЁЂPO43-ЃЛе§ШЗД№АИЃК
ЃЌПеМфЙЙаЭЮЊе§ЫФУцЬхаЮЃЌЦфжаЕЊдзгЕФМлВуЕчзгЖдЮЊ4ЃЌдгЛЏЗНЪНЮЊsp3дгЛЏЃЛClO4-ЪЧ5дзгЁЂ32МлЕчзгЕФРызгЃЌгыClO4-ЛЅЮЊЕШЕчзгЬхЕФЗжзгЛђРызггаCCl4ЁЂPO43-ЃЛе§ШЗД№АИЃК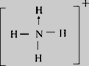 ЃЛе§ЫФУцЬхаЮ ЃЛ sp3дгЛЏ.ЃЛ CCl4ЁЂPO43- ЁЃ
ЃЛе§ЫФУцЬхаЮ ЃЛ sp3дгЛЏ.ЃЛ CCl4ЁЂPO43- ЁЃ
ЃЈ4ЃЉAКЌга1.5ИібЧЬњРызгЁЂ4ИіТШРызгЃЌBКЌга0.5ИібЧЬњРызгЁЂ4ИібѕРызгЁЂ4ИіЬњРызгЃЌдђИУбѕЛЏЮяжаFe2+ЁЂFe3+ЁЂO2-ЕФИіЪ§БШЮЊ1:2:4ЃЛе§ШЗД№АИЃК1ЃК2ЃК4ЁЃ
ЃЈ5ЃЉІУОЇЬхОЇАћжаЫљКЌгаЕФЬњдзгЪ§ЮЊ8ЁС1/8+6ЁС1/2=4ЃЌІФЁЂІССНжжОЇАћжаЬњдзгЕФХфЮЛЪ§ЗжБ№ЮЊ8ЁЂ6ЃЌдђХфЮЛЪ§жЎБШЮЊ8ЃК6=4ЃК3ЃЌгЩОЇАћЭМПЩжЊЃЌІФаЭОЇЬхЬњОЇАћжаFeдзгЪ§ФПЮЊ8ЁС1/8+1=2ЃЌОЇАћжЪСПЮЊ![]() gЃЌІФаЭОЇЬхЬњЕФУмЖШЮЊdg/cm3ЃЌОЇАћЬхЛ§ЮЊ
gЃЌІФаЭОЇЬхЬњЕФУмЖШЮЊdg/cm3ЃЌОЇАћЬхЛ§ЮЊ![]() gЁТdg/cm3=
gЁТdg/cm3=![]() cm3ЃЌдђОЇАћРтГЄ=
cm3ЃЌдђОЇАћРтГЄ=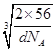 cmЃЌДІгкЬхЖдНЧЯпЩЯЕФдзгЯрСкЃЌдђ4r=
cmЃЌДІгкЬхЖдНЧЯпЩЯЕФдзгЯрСкЃЌдђ4r=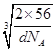 cmЁС
cmЁС![]() ЃЌЙЪr=
ЃЌЙЪr=![]() ЁС
ЁС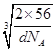 cm=
cm=![]() ЁС
ЁС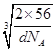 ЁС1010pmЃЌЙЪД№АИЮЊЃК4ЃЛ4ЃК3ЃЛ
ЁС1010pmЃЌЙЪД№АИЮЊЃК4ЃЛ4ЃК3ЃЛ![]() ЁС
ЁС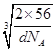 ЁС1010ЁЃе§ШЗД№АИЃК4 ЃЛ 4ЃК3ЃЛ .
ЁС1010ЁЃе§ШЗД№АИЃК4 ЃЛ 4ЃК3ЃЛ . 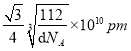 ЁЃ
ЁЃ


 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈ1ЃЉCO2ЪЧЮТЪвЦјЬхЃЌПЩгУNaOHШмвКЮќЪеЕУЕНNa2CO3ЛђNaHCO3ЁЃNa2CO3ЫзГЦДПМюЃЌвбжЊ25ЁцЪБЃЌCO32ЃЕквЛВНЫЎНтЕФЦНКтГЃЪ§Kh=2ЁС10-4mol/LЃЌЕБШмвКжаc(HCO3-)ЃКc(CO32Ѓ)=20ЁУ 1 ЪБЃЌШмвКЕФpH=______ЁЃ
ЃЈ2ЃЉЮЊСЫГ§ШЅвјЦїБэУцAg2SЃЌПЩВЩгУШчЯТЗНЗЈЃКдквЛИіТСжЦЕФШнЦїжаЗХШыЪГбЮШмвКЃЌНЋвјЦїНўШыЪГбЮШмвКЃЌЪЙвјЦїгыТСНгДЅСМКУаЮГЩдЕчГиЃЎЙ§вЛЖЮЪБМфЃЌвјЦїБэУцБфЮЊвјАзЩЋЃЌВЂЮХЕНГєМІЕАЕФЦјЮЖЃЌЙлВьЕНгаЩйСПАзЩЋаѕзДГСЕэЩњГЩЃЌдђдЕчГиЕФе§МЋЗДгІЮЊ______________________________ЃЌЧыНтЪЭГєМІЕАЦјЮЖаЮГЩЕФдвђЃЈгУРызгЗНГЬЪНБэЪОЃЉ________________________________________ЁЃ

ЃЈ3ЃЉ25 ЁцЃЌдк0.10 molЁЄLЃ1H2SШмвКжаЃЌЭЈШыHClЦјЬхЛђМгШыNaOHЙЬЬхвдЕїНкШмвКpHЃЌШмвКpHгыc(S2Ѓ)ЙиЯЕШчЭМ(КіТдШмвКЬхЛ§ЕФБфЛЏЁЂH2SЕФЛгЗЂ)ЁЃ
ЂйpHЃН11ЪБЃЌШмвКжаЕФc(H2S)ЃЋc(HSЃ)ЃН________molЁЄLЃ1ЁЃ
ЂкФГШмвККЌ0.040 molЁЄLЃ1M2ЃЋЁЂ0.10 molЁЄLЃ1H2SЃЌЕБШмвКpHЃН________ЪБЃЌMn2ЃЋПЊЪМГСЕэЁЃ[вбжЊЃКKsp(MS)ЃН5.6ЁС10Ѓ17]
ЃЈ4ЃЉNa2S2O3ШмвКГЃзїЮЊБъзМвКВтЖЈЮяжЪЕФзщГЩЁЃ
I.ШЁ3.92 gФГЬњЕФбѕЛЏЮяЃЌШмгкзуСПЯЁСђЫсЃЌВЂХфжЦГЩ100.0 mLШмвКЃЛ
II.ШЁ10.00 mLЫљЕУШмвКЃЌМгШызуСПKIШмвКЃЌЕЮМгМИЕЮжИЪОМСЃЛ
III.гУ0.2000 mol L-1ЕФNa2S2O3БъзМШмвКЕЮЖЈЃЌжиИД2ЁЋ3ДЮЃЌЦНОљЯћКФБъзМвК20.00mLЁЃ
вбжЊЃКI2+2S2O32-= S4O62-+2I-ЁЃдђЃК
ЂйВНжшII ЫљгУжИЪОМСЕФУћГЦЮЊ____________ЃЛХаЖЯДяЕНЕЮЖЈжеЕуЕФВйзїКЭЯжЯѓ___________________ЁЃ
ЂкИУЬњЕФбѕЛЏЮяЕФЛЏбЇЪНЮЊ______________ЁЃ
ЃЈ5ЃЉГЃЮТЯТЃЌЯђ20 mL 0.2 mol /L H2AШмвКжаЕЮМг0.2 mol /L NaOHШмвКЁЃгаЙиЮЂСЃЕФЮяжЪЕФСПБфЛЏШчЩЯЭМЃЌЦфжаШ§ЬѕЯпДњБэЕФЪЧA2ЃЁЂH2AКЭHAЃХЈЖШБфЛЏЕФЧњЯпЃЌИљОнЭМЪОЃЌЕБV(NaOH)ЃН20 mLЪБЃЌШмвКжаNaЃЋЁЂHAЃЁЂ A2ЃЁЂ H2AЫФжжЮЂСЃХЈЖШДѓаЁЙиЯЕЃК__________________________________ЁЃШмвКЯд_______адЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈе§ШЗЕФЪЧ
A. C2H2ЗжзгжаІвМќгыІаМќЕФЪ§ФПБШЮЊ1ЃК1ЧвІвМќБШІаМќжиЕўГЬЖШДѓЃЌаЮГЩЕФЙВМлМќЧП
B. SO2КЭCS2ОљЮЊVаЮЕФМЋадЗжзг
C. ЂйH3O+ Ђк[Cu(NH3)4]2+ ЂлCH3COO- ЂмNH3 ЂнCH4жаДцдкХфЮЛМќЕФЪЧЂйЂкЂн
D. SiF4КЭSO32-ЕФжааФдзгОљЮЊsp3дгЛЏ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНЋ1.66 gЬМЫсФЦКЭЧтбѕЛЏФЦЕФЙЬЬхЛьКЯЮяЭъШЋШмгкЫЎХфГЩЯЁШмвКЃЌШЛКѓЯђИУШмвКжаж№ЕЮМгШы1 mo1ЁЄL-1ЕФбЮЫсЃЌЫљМгШыбЮЫсЕФЬхЛ§гыВњЩњCO2ЕФЬхЛ§ЃЈБъзМзДПіЃЉЙиЯЕШчЭМЫљЪОЁЃ
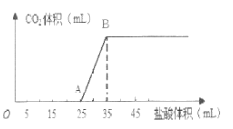
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉOAЖЮЪЧбЮЫсгыNa2CO3КЭNaOHЗЂЩњЕФЗДгІЃЌЗДгІКѓШмвКжаЕФШмжЪга_________ЃЈЬюЛЏбЇЪНЃЉЁЃ
ЃЈ2ЃЉЕБЕНДяBЕуЪБЃЌВњЩњCO2ЕФЬхЛ§ЮЊ ________________ mLЃЈБъзМзДПіЃЉЁЃ
ЃЈ3ЃЉМЦЫудЛьКЯЮяжаNa2CO3ЕФжЪСПЗжЪ§________________ЁЃЃЈаДГіБивЊМЦЫуЙ§ГЬЃЌНсЙћБЃСє3ЮЛгааЇЪ§зжЃЉ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЙигкЩњЮяДѓЗжзгЕФа№ЪіжаЃЌДэЮѓЕФЪЧ
A. ЕААзжЪЪЧвдАБЛљЫсЮЊЛљБОЕЅЮЛЙЙГЩЕФЩњЮяДѓЗжзг
B. КЫЫсЪЧДЂДцвХДЋаХЯЂЁЂПижЦЕААзжЪКЯГЩЕФЩњЮяДѓЗжзг
C. ЕэЗлЁЂИЮЬЧдЁЂЯЫЮЌЫиКЭКЫЬЧЖМЪЧЩњЮяДѓЗжзг
D. ЖрЬЧЁЂЕААзжЪЁЂКЫЫсЕШЪЧвдЬМСДЮЊЙЧМмЕФЩњЮяДѓЗжзг
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊ2-ЖЁЯЉгаЫГЁЂЗДСНжжвьЙЙЬхЃЌдкЦфЬѕМўЯТПЩжжЦјЬхДІгкЦНКтЃЌ

 (g)+H2(g)ЁњCH3CH2CH2CH3(g) ЁїH=-118.9kJ/molЃЛ
(g)+H2(g)ЁњCH3CH2CH2CH3(g) ЁїH=-118.9kJ/molЃЛ
ЯТСаЫЕЗЈе§ШЗЕФЪЧ
A. ЫГ-2-ЖЁЯЉБШЗД-2-ЖЁЯЉЮШЖЈ
B. МгбЙКЭНЕЮТгаРћгкЦНКтЯђЩњГЩЫГ-2- ЖЁЯЉЗДгІЗНЯђвЦЖЏ
C. .ЫГ-2-ЖЁЯЉЕФШМЩеШШБШЗД-2-ЖЁЯЉаЁ
D. ЗД-2-ЖЁЯЉЧтЛЏЕФШШЛЏбЇЗНГЬЪНЮЊ (g)+H2(g)ЁњCH3CH2CH2CH3(g) ЁїH=-114.7kJ/mol
(g)+H2(g)ЁњCH3CH2CH2CH3(g) ЁїH=-114.7kJ/mol
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯђФГШмвКжаЕЮМгбЮЫсЫсЛЏЃЌдйЕЮМгBaCl2ШмвКЃЌВњЩњАзЩЋГСЕэЁЃдђИУШмвКвЛЖЈга
A. CO32-B. NO3-C. SO32-D. SO42-
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЕБЙтЪјЭЈЙ§ЪБЃЌФмЙлВьЕНЖЁДяЖћаЇгІЕФЪЧ
A. ЯЁСђЫсB. CuSO4 ШмвКC. ОЦОЋШмвКD. Fe(OH)3НКЬх
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаРызгЗНГЬЪНЪщаДе§ШЗЕФЪЧ (ЁЁЁЁ)
A. ЯђЫЎжаЭЈШыТШЦјЃКCl2ЃЋH2O![]() 2HЃЋЃЋClЃЃЋClOЃ
2HЃЋЃЋClЃЃЋClOЃ
B. ЯђТШЛЏЬњШмвКжаМгШыЭЃК2Fe3ЃЋЃЋ3Cu===2FeЃЋ3Cu2ЃЋ
C. NH4HCO3ШмвКгыЙ§СПKOHХЈШмвКЙВШШЃКNH4ЃЋЃЋOHЃ![]() NH3ЁќЃЋH2O
NH3ЁќЃЋH2O
D. ЯђЖўбѕЛЏУЬжаЕЮМгХЈбЮЫсВЂМгШШЃКMnO2ЃЋ4HЃЋЃЋ2ClЃ![]() Mn2ЃЋЃЋCl2ЁќЃЋ2H2O
Mn2ЃЋЃЋCl2ЁќЃЋ2H2O
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com