
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������980mL��0.1mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������980mL��0.1mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У����� ��1������������Һ��ʵ���������ѡ������������Ͳ���Ҫʹ�õ�������
��2������c=$\frac{1000�Ѧ�}{M}$����ŨH2SO4�����ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ�����ʵ��������������Ũ����������
��3������ʹ�ò������IJ�������������;��
��4���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�������������裻
��5������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺��1������ʵ������980mL����ƿ����Ӧѡ��1000mL����ƿ�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ��ת�Ƶ�1000mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ����ṩ��������֪����Ҫ�����У�1000mL����ƿ������Ҫ�������У�����ƿ����ҩ�ף���������ƽ��
�ʴ�Ϊ���ڢܢޣ�1000mL����ƿ����������
��2��ŨH2SO4�����ʵ���Ũ��c=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L��
����ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL�����У�
xmL��18.4mol/L=1000mL��0.1mol/L����ã�x��5.4
�ʴ�Ϊ��5.4mL��
��3�������ƹ����У��ܽ��������ƹ���ʱ����������;�ǣ����裬�����ܽ⣻����Һʱ����������;���������ʴ�Ϊ�����裬�����ܽ⣻����Һʱ����������;��������
��4���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��������Ϊ���ڢ٢ۢ�ݢޢߢܣ��ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��5����ϴ����ȡŨ�������Ͳ������ϴ��Һת�Ƶ�����ƿ�У��ᵼ������ƫ�࣬��Ũ��ƫ�ߣ��ʢ���ȷ��
��δ��ϡ�ͺ��������Һ��ȴ�����¾�ת�Ƶ�����ƿ�У�����ȴ����Һ���ƫС����Ũ��ƫ�ߣ��ʢ���ȷ��
��ת��ǰ������ƿ�к�����������ˮ����Ũ����Ӱ�죬�ʢ۴���
��δϴ��ϡ��Ũ����ʱ�ù����ձ��Ͳ��������ᵼ�����ʵ���ʧ����Ũ��ƫ�ͣ��ʢܴ���
�ݶ���ʱ�����ӿ̶��ߣ�����Һ���ƫС��Ũ��ƫ�ߣ��ʢ���ȷ��
�ʴ�Ϊ���٢ڢݣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2��CO������ȼ | |
| B�� | ����أ��Ȼ��ƣ���Ũ��Һ���½ᾧ | |
| C�� | MgSO4��MgCl2�������ɡ����� | |
| D�� | ���ᣨ��ȩ����������������ͭ��Һ���ȣ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �Թ����Լ� | �Թ������� | �� �� | |
| A | �����ữ��BaCl2��Һ | ���ɰ�ɫ���� | ��ɫ����ΪBaSO3 |
| B | Ʒ����Һ | ��Һ��ɫ | SO2����Ư���� |
| C | ��ɫʯ����Һ | ��Һ��� | SO2ˮ��Һ������ |
| D | ����KMnO4��Һ | ��ɫ��ȥ | SO2���л�ԭ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ����Cl2 | B�� | һ����SO2 ��NO | ||
| C�� | ������NO2 | D�� | һ����SO2��������NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 11.2g | B�� | 16.8g | C�� | 22.4g | D�� | 33.5g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
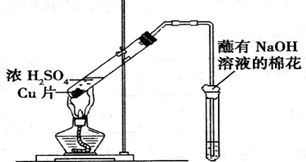
 ʵ����Ҫ��NaCl��������500mL 0.2mol•L-1NaCl��Һ���ش��������⣺
ʵ����Ҫ��NaCl��������500mL 0.2mol•L-1NaCl��Һ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ڵ�IIIA�� | B�� | �������ڵ�VA�� | ||
| C�� | �������ڵ�IIIB�� | D�� | �������ڵ�VB�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
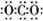 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
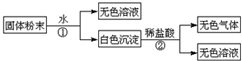
| ��� | ��ѧʽ |
| �� | |
| �� | |
| �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com