
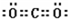 ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O������ A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3���ݴ˽��н��
��� �⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�������Ӧ���������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��AΪ̼Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪþԪ�أ�EΪCrԪ�أ�
��1��AΪ̼Ԫ�ء�BΪ��Ԫ�ء�CΪ��Ԫ�أ�ͬ����������ҵ�һ����������Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����������ͣ���Ԫ�ص�һ�����ܸ������ڵ�Ԫ�صģ����Ե�һ��������С�����˳��Ϊ��C��O��N��
�ʴ�Ϊ��C��O��N��
��2��BΪ��Ԫ�أ����⻯��ΪNH3�������к���3��N-H����Nԭ����1�Թ¶Ե��Ӷԣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ռ乹��Ϊ�����ͣ�
�ʴ�Ϊ�������ͣ�sp3��
��3��������AC2��CO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ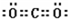 ��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
�ʴ�Ϊ��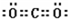 ��N2O��
��N2O��
��4��EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ��[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��[Cr��NH3��4��H2O��2]Cl3��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ��漰�ṹ����Խλ�ù�ϵ��Ԫ�������ɡ�����ʽ���������Ų�����������ӻ����ۡ����ӽṹ�����٣������ʽṹ���ۺ�����Ŀ���Ƕ�ѧ���ۺ������Ŀ��飬�⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �û��������NaHCO3��Һ��Ӧ�ų�CO2���� | |
| B�� | 1 mol�û�������������3 mol NaOH��Ӧ | |
| C�� | �û��������ʹ����KMnO4��Һ��ɫ | |
| D�� | �û���������ڹ�������Cl2����ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ���ݢ��� | B�� | ���ܢߢ��� | C�� | ���ܢݢޢ��� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ�����ϩ | B�� | ������ܽ�ȵIJⶨ | ||
| C�� | ʵ��������ʯ�� | D�� | ú�ĸ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ� | B�� | ��ȩ | C�� | ���� | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ȼ����Ϊ285.8kJ•mol-1��������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g���T2H2O��l����H=-285.8 kJ•mol-1 | |
| B�� | ��֪�к���Ϊ57.3 kJ•mol-1������1L1mol•L-1�����뺬1molNaOH��Һ��ϣ��ų�������ҪС��57.3kJ | |
| C�� | Ba��OH��2•8H2O��s��+2NH4Cl��s���TBaCl2��s��+2NH3��g��+10H2O��l����H��0 | |
| D�� | ������������������Ʒֱ���ȫȼ�գ����߷ų��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol•L-1CH3COOH��Һ�У�c��CH3COO-����c��H+�� | |
| B�� | 0.1mol•L-1NH4Cl��Һ�У�c��NH+��+c��H+��=c��Cl-��+c��OH-�� | |
| C�� | 0.1mol•L-1Na2CO3��Һ��0.1mol•L-1NaHCO3��Һ��������������Һ��c��CO32-��+2c��OH-��=c��HCO3-��+3c��H2CO3��+2c��H+�� | |
| D�� | Ũ�Ⱦ�Ϊ0.1mol•L-1NH4Cl��CH3COONH4��NH4HSO4��Һ�У�c��NH4+���Ĵ�С˳��CH3COONH��NH4Cl��NH4HSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
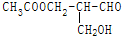 �й�ѧ���ԣ��������з�Ӧ�����ɵ��л�����ѧ���Ե��ǣ�������
�й�ѧ���ԣ��������з�Ӧ�����ɵ��л�����ѧ���Ե��ǣ�������| A�� | ����ᷢ��������Ӧ | B�� | ��NaOHˮ��Һ���� | ||
| C�� | ��������Һ���� | D�� | ����Br2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
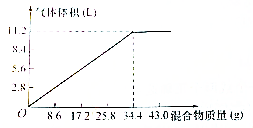 ��100mL NaOH��Һ�м���NH4NO3�ͣ�NH4��2SO4�̶�����ﹲ�ȣ�����������������Ͳ��������������ѻ���ɱ�װ���Ĺ�ϵ��ͼ��ʾ���ٶ����ɵ�NH3ȫ���ݳ������Լ��㣺
��100mL NaOH��Һ�м���NH4NO3�ͣ�NH4��2SO4�̶�����ﹲ�ȣ�����������������Ͳ��������������ѻ���ɱ�װ���Ĺ�ϵ��ͼ��ʾ���ٶ����ɵ�NH3ȫ���ݳ������Լ��㣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com