
�ж�����˵���Ƿ���ȷ��������ȷ��˵�����ɡ�
��Ԫ������Ȼ���еĴ�����ʽ��ԭ�ӡ����ӻ�����
���ڻ�ѧ�仯�У����ӿ����ٷ֣����Ӻ�ԭ�Ӳ������ٷ�
��ͬһ��Ԫ�ؿ����ж��ֲ�ͬԭ�ӣ�ͬһ��ԭ��Ҳ�����γɲ�ͬ������
��ԭ�����ԭ���γɵ��������ԭ�������������
����ͬһ��Ԫ����ɵ�����һ����ͬһ������
�����ʵĻ�ѧ����һ�����ɷ��ӱ��ֵ�
�����������������͵���������ͬ������һ����ͬһ������
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F��G�����л���������ǵĹ�ϵ��ͼ��ʾ��
(1)������C�ķ���ʽ��C7H8O��C��FeCl3��Һ����ɫ��C�ı����ϵ�һ�����ֻ�����֣���C�Ľṹ��ʽΪ ��
(2)DΪһֱ�����������Է��������Ȼ�����C��С20�����ܸ�NaHCO3��Ӧ�ų�CO2����D�ķ���ʽΪ ��D���еĹ����������� ��
(3)��Ӧ�ٵĻ�ѧ����ʽ�� ��
(4)���㻯����B����A������ͬ�����ŵ�A��ͬ���칹�壬ͨ����Ӧ�ڻ�����B������E��F��F���ܵĽṹ��ʽ�� ��
(5)E���ܵĽṹ��ʽ�� ��
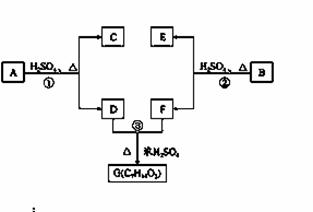
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪C(s)��H2O(g)��CO(g)��H2(g) ��H��akJ��mol��1
2C(s)��O2(g)��2CO(g) ��H����220kJ��mol��1
H��H��O��O��O��H���ļ��ֱܷ�Ϊ436��496��462kJ��mol��1,��aΪ( )
A����332 B����118 C����350 D����130
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����
A�������ԭˮ����ȵ��ˮ��������ܻ�����������
B��������ˮ(��NH4+��NH3)���û�ѧ��������绯ѧ����������
C��ij�ֹ�ѧ��⼼�����м��ߵ������ȣ��ɼ�����ϸ��(V=10��12L)�ڵ�����Ŀ����ӣ��ݴ˿�����ü�⼼���ܲ�����ϸ����Ũ��ԼΪ10��12��10��11mol ·L��1��Ŀ�����
D�������������Ӽ״��û��ȼ�ϵ���ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о�С��Ϊ��̽��һ����������X(��������Ԫ��)����ɺ����ʣ���Ʋ��������ʵ�飺
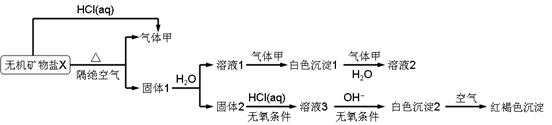 |
��ȡ10.80gX�ڶ��������м�������ȫ�ֽ⣬�õ�6.40g����1.��ش��������⣺
(1)������ɫ����1�н���Ԫ�ص�ԭ�ӽṹʾ��ͼ_______��д������ĵ���ʽ_______��
(2)X�Ļ�ѧʽ��______���ڶ��������м���X����ȫ�ֽ�Ļ�ѧ��Ӧ����ʽΪ_______��
(3)��ɫ����2�ڿ����б�ɺ��ɫ������ԭ����_______(�û�ѧ��Ӧ����ʽ��ʾ)��
(4)һ�������£�����������1�е�ij�ֳɷֿ��ܷ���������ԭ��Ӧ��д��һ�����ܵĻ�ѧ��Ӧ����ʽ_______�������ʵ�鷽����֤�÷�Ӧ�IJ���_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���ÿ�����4��ѡ����������1��ѡ�������3��ѡ�����ڲ�ͬ�ķ��࣬�뽫������ѡ�����ż���ѡ���������±���
| ��� | ��ѡ�� | ����ѡ ����� | ��ѡ���� |
| (1) | A.S2�� B��I�� C��Fe��D��SO | ||
| (2) | A.HCl B��CO2 C��NH3 | ||
| (3) | A.����B����Һ C.������D���к� | ||
| (4) | A.KMnO4��B��Al2(SO4)3 C.KClO3�� D��K2HPO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Na��S���ʼ��仯��������������б�ŵĻ�ѧ����ʽ��
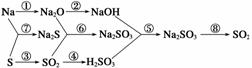
�����йط�Ӧ�Ļ�ѧ����ʽΪ
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵķ�����ȷ���� (����)
����� ���ᡡ�� ���Ρ� �� ���������������������
A��Na2CO3 H2SO4����NaOH���� SO2������ CO2
B��NaOH HCl NaCl Na2O NO
C��KOH HNO3 CaCO3 CaO Mn2O7
D��NaOH HCl CaF2 Na2O2 SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ���� (����)
A����������ˮʱ��Cl2��H2O2H����Cl����ClO��
B���ö��Ե缫��ⱥ���Ȼ�����Һ��2Cl����2H2O===H2����Cl2����2OH��
C����Fe(NO3)3��Һ�м��������HI��Һ��2Fe3����2I��===2Fe2����I2
D��Na2SO3��Һʹ����KMnO4��Һ��ɫ��5SO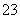 ��6H����2MnO
��6H����2MnO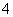 ===5SO
===5SO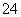 ��2Mn2����3H2O
��2Mn2����3H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com