
(8·Ö)¹żĢ¼ĖįÄĘŗĶŃĪĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2Na2CO4+4HCl=4NaCl+2CO2”ü+O2”ü+2H2O£¬ÉĢĘ·¹żĢ¼ĖįÄĘÖŠŅ»°ć¶¼ŗ¬ÓŠNa2CO3£¬ĪŖĮĖ²ā¶ØĖüµÄ“æ¶Č£¬Č”Ņ»¶ØĮæµÄѳʷŗĶŃĪĖį·“Ó¦£¬Ķعż²āĮæ²śÉśµÄŃõĘųµÄĢå»ż£¬æÉŅŌ¼ĘĖć³ö¹żĢ¼ĖįÄʵÄŗ¬Į攣
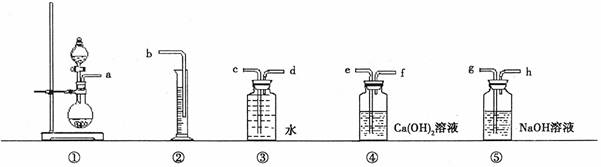
(1)øł¾ŻÉĻĶ¼Ģį¹©µÄŅĒĘ÷×°ÖĆ£¬×é×°Ņ»Ģײā¶ØÉĢĘ·¹żĢ¼ĖįÄĘ“æ¶ČµÄŹµŃé×°ÖĆ£¬ÕāŠ©×°ÖƵÄĮ¬½ÓĖ³ŠņŹĒ(Ģī½ÓæŚ×ÖÄø)£ŗ ”£
(2)×°ÖĆ¢ÜµÄ×÷ÓĆ£ŗ
ӣ
(3)Čē¹ūŹµŃ鏱£¬³ĘČ”wgѳʷŗĶ¹żĮæŃĪĖį·“Ó¦ŗó£¬ĖłµĆŃõĘųµÄĢå»ż(±ź×¼×“æö)ĪŖVmL£¬Ōņ¼ĘĖć“Ėѳʷ“æ¶ČµÄ±ķ“ļŹ½ĪŖ ”£
(4)ij“ĪŹµŃ飬³ĘČ”O.9gѳʷ½ųŠŠ²ā¶Ø”£ŹµŃéŹŅĻÖÓŠ50mL£¬100mL£¬150mLČżÖÖ¹ęøńµÄĮæĶ²£¬Ó¦Ń”ÓĆ¹ęøńĪŖ µÄĮæĶ²½ųŠŠŹµŃ锣

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ĪĀ¶Č”ę Čܽā¶Č ČÜÖŹ g/100gĖ® |
10 | 20 | 30 | 40 | 50 | 60 | 70 |
| NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 37.8 |
| NH4HCO3 | 15.8 | 21.0 | 27.0 | ||||
| NaHCO3 | 8.2 | 9.6 | 11.1 | 12.7 | 14.4 | 16.4 | |
| NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | 60.2 |
| c”ĮV1 ”Į10-3”ĮM(Na2CO3)g |
| Gg |
| c”ĮV1 ”Į10-3”ĮM(Na2CO3)g |
| Gg |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com