
| A�� | O.1mol/LKHC03��Һ��O��1mol/L KOH��Һ�������ϣ�������Һ�У�c��K+����c��CO32-����c��HCO3-����c��OH-�� | |
| B�� | pH=1��NaHSO4��Һ��c��H+���Tc��SO42-��+c��OH-�� | |
| C�� | 20ml O��lmol/L CH3COONa��Һ��lOmLO��lmol/L HCl��Һ��Ϻ���Һ�����ԣ�������Һ�У�C��Cl-������CH3COO-����c��H+����c��CH3COOH�� | |
| D�� | pH=2��H2C2O4��Һ��pH=l2��NaOH��Һ���������ϣ�c��Na+��+c��H+��=c��OH-��+c��HC2O4-�� |
���� A��O.1mol/LKHC03��Һ��O��1mol/L KOH��Һ�������ϣ�������Һ��̼�����Һ���ݴ˻ش�
B��pH=1��NaHSO4��Һ������Ũ�Ⱥ�������Ũ���Լ����������Ũ����ȣ����ݵ���غ����ش�
C��20ml O��lmol/L CH3COONa��Һ��lOmLO��lmol/L HCl��Һ��Ϻ�õ����Ǵ���ʹ����ƵĻ�����Һ�����ԣ�������Һ��������ӵ�ˮ��С�ڴ���ĵ���̶ȣ�
D��������Һ�еĵ���غ�֪ʶ���ش�
��� �⣺A��O.1mol/LKHC03��Һ��O��1mol/L KOH��Һ�������ϣ�������Һ��̼�����Һ��������Һ�У�c��K+����c��CO32-����c��OH-����c��HCO3-������A����
B��pH=1��NaHSO4��Һc��Na+��=c��H+���Tc��SO42-��+�����ݵ���غ�c��Na+��+c��H+���T2c��SO42-��+c��OH-������c��H+���Tc��SO42-��+c��OH-������B��ȷ��
C��20ml O��lmol/L CH3COONa��Һ��lOmLO��lmol/L HCl��Һ��Ϻ�õ����Ǵ��ᡢ�Ȼ��ƺʹ����ƵĻ�����Һ�����ԣ�������Һ��������ӵ�ˮ��С�ڴ���ĵ���̶ȣ���ʱc��Cl-����c��CH3COO-������C����
D��pH=2��H2C2O4��Һ��pH=l2��NaOH��Һ���������ϣ������ڵ���غ㣺c��Na+��+c��H+��=c��OH-��+c��HC2O4-��+2c��C2O42-������D����
��ѡB��
���� �����漰�ε�ˮ��ԭ����Ӧ�á�����Ũ�ȴ�С�Ƚϵ�֪ʶ��ע�����غ㡢�����غ��Լ������غ��Ӧ���ǹؼ����Ѷ��еȣ�


 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4 C2H6 | B�� | C2H4 C3H6 | C�� | C2H4 C3H4 | D�� | C2H6 C3H6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
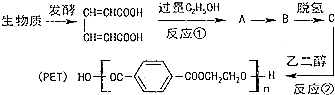
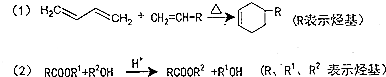
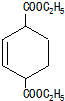 ��
��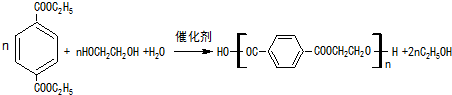 ��
��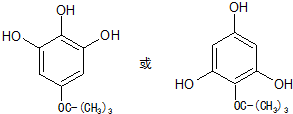 ����дһ�֣�
����дһ�֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
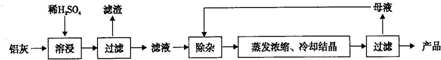
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 2.7 |
| ��ȫ����ʱ | 5.2 | 9.7 | 3.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��С�մ�����θ����� | |
| B�� | ��ʯ����ʳƷ����� | |
| C�� | ��ĥ��ʯ��ָ���� | |
| D�� | �����ظ���ؼ���˾���Ƿ�ƺ�ݳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ͷ�Ӧ�¶� | B�� | �ӳ���Ӧʱ�� | C�� | �������ʯ | D�� | ��ˮϡ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
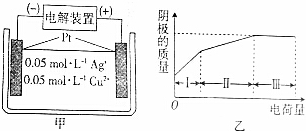
| A�� | ���������������������� | |
| B�� | ���������Һ��pH������ | |
| C�� | ��������������������ų� | |
| D�� | �������������������������2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com