
 ��ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�x��y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�������������ͼ��ʾ����ش��������⣺
��ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�x��y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�������������ͼ��ʾ����ش��������⣺

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�X��Y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ���벬�缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�����ش��������⣺
����ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�X��Y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ���벬�缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��
����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
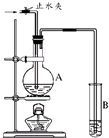 ����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��
����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ�����ص�һ��ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
(10��)��ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�x��y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S�����պϿ���S����ֱͨ����һ��ʱ�������������ͼ��ʾ��
��ش��������⣺
(1)�����Դ������������xΪ���� ��
(2)����ֽ��C�˸������۲쵽���������� �� �� ��?
(3)д���缫��Ӧʽ��B�缫������ ������������������ ������ ��?
(4)�����һ��ʱ���A��B�о��������Χ�缫����ʱ�жϿ���S���պϿ���S����������Ƶ�ָ���Ƿ���ƫת���������� (�ƫת����ƫת��)��?
(5)��������ָ��ƫת��д���йصĵ缫��Ӧ(��ָ�롰��ƫת�������ⲻ�ػش�)��
������ ���������� �� ���� �������� ��?
��������ָ�벻ƫת����˵������(��ָ�롰ƫת�������ⲻ�ػش�)������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com