
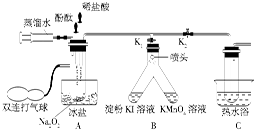
 2H2O��O2��
2H2O��O2��

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A�������¶ȣ���Һ��pH���� |
| B��c(OH��)��c (H��)��c (HCO3��)��2 c (H2CO3) |
| C����������NaOH���壬c (CO32�D)��c (Na��)������ |
| D��c (Na��) > c (CO32�D) > c (HCO3�D) > c(OH�D) > c (H��) |
 Ni + 2NaCl����������Ӧʽ��_____��
Ni + 2NaCl����������Ӧʽ��_____���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2.1 g | B��3.6 g |
| C��7.2 g | D����ȷ�� |
�鿴�𰸺ͽ���>>
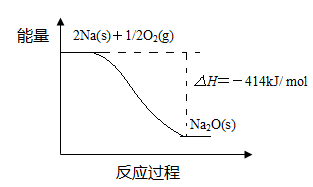
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
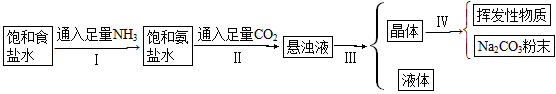
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
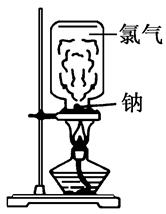

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
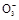
 BaCO3��+H2O)
BaCO3��+H2O)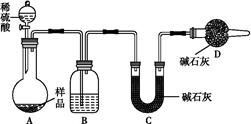
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���±���
���±��� | H2CO3 | Ka1=4.3��10-7 | H2C2O4 | Ka1=5.6��10-2 |
| Ka2=5.6��10-11 | Ka2=5.42��10-5 |
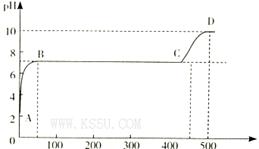
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���մ��С�մ������ᷴӦ�������������Ʒ�Ӧ |
| B��������ϡ���ᷴӦ�����ɵ����Ķ�����̼���ĵ��մ�����ʵ�����С�մ�� |
| C����С�մ���Һ�е�������������Һ���������մ���Һ�е�������������Һ���ֳ��� |
| D�����������մ�С�մ�ֱ�������ϡ���ᷴӦ��С�մ�����Ķ�����̼�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com