
Ԫ�ص��ʼ��仯�����й㷺��;����������ڱ��е�������Ԫ�����֪ʶ�ش��������⣺
(1)��ԭ������������˳��(ϡ���������)������˵����ȷ����________��
a��ԭ�Ӱ뾶�����Ӱ뾶����С
b�������Լ������ǽ�������ǿ
c���������Ӧ��ˮ������Լ�����������ǿ
d�����ʵ��۵㽵��
(2)ԭ�������������������������ͬ��Ԫ������Ϊ________�������������ļ���������________��
(3)��֪��
| ������ | MgO | Al2O3 | MgCl2 | AlCl3 |
| ���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
| �۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ����__________________________________��
����ʱ�����Al2O3�������AlCl3��ԭ����______________________________��
(4)�����(�۵�1410 ��)�����õİ뵼����ϡ��ɴֹ��ƴ���������£�
Si(��)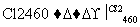 SiCl4
SiCl4 SiCl4(��)
SiCl4(��)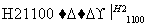 Si(��)
Si(��)
д��SiCl4�ĵ���ʽ��________________����������SiCl4�ƴ���ķ�Ӧ�У����ÿ����1.12 kg����������a kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(5)P2O5�Ƿ������Ը�������������岻����Ũ����������P2O5�������________��
a��NH3 ��b��HI c��SO2 d��CO2
(6)KClO3������ʵ������O2�������Ӵ�����400 ��ʱ�ֽ�ֻ���������Σ�����һ�����������Σ���һ���ε��������Ӹ�����Ϊ1��1��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(1)b��(2)벡�Na��(��������)
(3)MgO���۵�ߣ�����ʱ�ķѸ�����Դ�����������ɱ�
AlCl3�ǹ��ۻ��������̬�ѵ���
(4)
SiCl4(g)��2H2(g)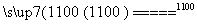 Si(s)��4HCl(g)
Si(s)��4HCl(g)
��H����0.025a kJ��mol��1
(5)b
(6)4KClO3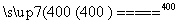 KCl��3KClO4
KCl��3KClO4
[����] (1)��ϡ�������⣬��������Ԫ����ԭ�������ĵ���ԭ�Ӱ뾶��С�������Ӱ뾶��һ����С����r(Na��)��r(Cl��)��a����ͬһ���ڵ�����Ԫ����ԭ�������ĵ����������Լ������ǽ�������ǿ��b��ȷ��ͬ��������Ԫ�ش������ң�����������Ӧ��ˮ������Լ�����������ǿ��c�����ʵ��۵㲻һ�����ͣ���Na���۵����Mg��Al�ȵ��۵㣬d����(2)��������Ԫ�ص�ԭ�Ӻ������������Ӳ㣬����������Ϊ8���ʸ�Ԫ��ԭ�������ĵ�����ҲΪ8����Ԫ��Ϊ벣������ӵ�������Խ������Ӧ���ʵĻ�ԭ��Խǿ��Ԫ�صĽ�����Խǿ�����������н�������ǿ��Ԫ����Na�����Na����������������(3)��������ݱ�֪��MgO���۵��MgCl2���۵�ߣ�MgO����ʱ�ķѸ�����Դ�����ӳɱ���AlCl3�ǹ��ۻ��������̬ʱ�����룬�ѵ��磬��ұ���������õ��AlCl3�ķ�����(4)SiCl4���ڹ��ۻ���������ʽΪ ����SiCl4�ƴ���Ļ�ѧ����ʽΪSiCl4(l)��2H2(g)
����SiCl4�ƴ���Ļ�ѧ����ʽΪSiCl4(l)��2H2(g)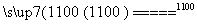 Si(s)��4HCl(g)������1.12 kg��40 mol��������a kJ������������1 mol����������0.025a kJ������(5)NH3�Ǽ������壬�Ȳ�����Ũ������Ҳ������P2O5���HI�Ǿ��л�ԭ�Ե��������壬����P2O5�����������Ũ������SO2��CO2������Ũ������������P2O5������Ϸ�������ȷ��Ϊb��(6)KClO3(Cl�Ļ��ϼ�Ϊ��5��)���ȷֽ����ɵ�����������KCl����һ������������Ԫ�صĻ��ϼ۱���ȣ�5�۸ߣ�����Ϊ��6�ۻ�7�ۣ���Ϊ��6�ۣ��γɵ������������Ӹ����Ȳ�����Ϊ1��1��ֻ���ǣ�7�ۣ��ʸú�������ΪKClO4���ݴ˿�д���÷�Ӧ�Ļ�ѧ����ʽ��
Si(s)��4HCl(g)������1.12 kg��40 mol��������a kJ������������1 mol����������0.025a kJ������(5)NH3�Ǽ������壬�Ȳ�����Ũ������Ҳ������P2O5���HI�Ǿ��л�ԭ�Ե��������壬����P2O5�����������Ũ������SO2��CO2������Ũ������������P2O5������Ϸ�������ȷ��Ϊb��(6)KClO3(Cl�Ļ��ϼ�Ϊ��5��)���ȷֽ����ɵ�����������KCl����һ������������Ԫ�صĻ��ϼ۱���ȣ�5�۸ߣ�����Ϊ��6�ۻ�7�ۣ���Ϊ��6�ۣ��γɵ������������Ӹ����Ȳ�����Ϊ1��1��ֻ���ǣ�7�ۣ��ʸú�������ΪKClO4���ݴ˿�д���÷�Ӧ�Ļ�ѧ����ʽ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں����ܱ�������ͨ��X��������Ӧ��2X(g) Y(g)���¶�T1��T2��X�����ʵ���Ũ��c(X)��ʱ��t�仯��������ͼ��ʾ������������ȷ����(����)
Y(g)���¶�T1��T2��X�����ʵ���Ũ��c(X)��ʱ��t�仯��������ͼ��ʾ������������ȷ����(����)
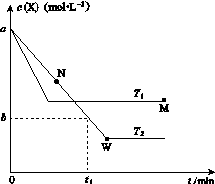
A���÷�Ӧ���е�M��ų����������ڽ��е�W��ų�������
B��T2�£���0��t1ʱ���ڣ�v(Y)��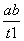 mol��L��1��min��1
mol��L��1��min��1
C��M�������Ӧ����v������N����淴Ӧ����v��
D��M��ʱ�ټ���һ����X��ƽ���X��ת���ʼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺
(1)��֪AX3���۵�ͷе�ֱ�Ϊ��93.6 ���76 �棬AX5���۵�Ϊ167 �档����ʱAX3������X2��Ӧ����1 mol AX5���ų�����123.8 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
(2)��ӦAX3(g)��X2(g)  AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
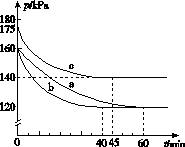
����ʽ����ʵ��a�ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)��______________________��
��ͼ��3��ʵ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)�ɴ�С�Ĵ���Ϊ____________(��ʵ�����)����ʵ��a��ȣ���������ı��ʵ���������ж������ǣ�b________________________________________________��c____________________________________________��
����p0��ʾ��ʼʱ��ѹǿ��p��ʾƽ��ʱ��ѹǿ������ʾAX3��ƽ��ת���ʣ������ı���ʽΪ______________��ʵ��a��c��ƽ��ת���ʣ���aΪ________����cΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֡�
(1)���ü������ԭ���������֪��
CH4(g)��4NO2(g)===4NO(g)��CO2(g)��2H2O(g)����H����574 kJ/mol
CH4(g)��4NO(g)===2N2(g)��CO2(g)��2H2O(g)
��H����1160 kJ/mol
��CH4��NO2��ԭΪN2���Ȼ�ѧ����ʽΪ__________________________________________________________��
(2)����NH3����ԭ���������ֳ�SCR�������ü�����ĿǰӦ����㷺���������������ѳ���������Ӧ�Ļ�ѧ����ʽΪ2NH3(g)��NO(g)��NO2(g)
 2N2(g)��3H2O(g)����H<0��
2N2(g)��3H2O(g)����H<0��
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ��____________________________________________________(д��1������)��
(3)����ClO2�������������ת���������£�
NO NO2
NO2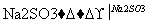 N2
N2
��֪��Ӧ��Ļ�ѧ����ʽΪ2NO��ClO2��H2O===NO2��HNO3��HCl����Ӧ��Ļ�ѧ����ʽ��____________________________________��������11.2 L N2(��״��)��������ClO2________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CaSO4����O2��ȼ��CO��Ӧ���ȿ����ȼ��Ч�ʣ����ܵõ��ߴ�CO2����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ�������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��
��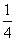 CaSO4(s)��CO(g)
CaSO4(s)��CO(g)
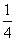 CaS(s)��CO2(g)��
CaS(s)��CO2(g)��
��H1����47.3 kJ��mol��1
��CaSO4(s)��CO(g) CaO(s)��CO2(g)��SO2(g)����H2����210.5 kJ��mol��1
CaO(s)��CO2(g)��SO2(g)����H2����210.5 kJ��mol��1
��CO(g)
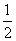 C(s)��
C(s)��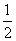 CO2(g)����H3����86.2 kJ��mol��1
CO2(g)����H3����86.2 kJ��mol��1
(1)��Ӧ2CaSO4(s)��7CO(g) CaS(s)��CaO(s)��6CO2(g) ��C(s)��SO2(g)�Ħ�H��________(�æ�H1����H2�ͦ�H3��ʾ)��
CaS(s)��CaO(s)��6CO2(g) ��C(s)��SO2(g)�Ħ�H��________(�æ�H1����H2�ͦ�H3��ʾ)��
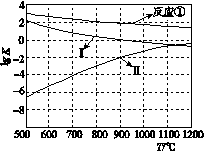
(2)��Ӧ�١��۵�ƽ�ⳣ���Ķ���lg K�淴Ӧ�¶�T�ı仯������ͼ��ʾ����ϸ���Ӧ�Ħ�H������lg K��T���߱仯���ɣ�
a��________�� b��________��
(3)��ʢ��CaSO4����պ����ܱ������г���CO����Ӧ����900 ��ﵽƽ�⣬cƽ��(CO)��8.0��10��5mol��L��1������CO��ת����(���Ը���Ӧ�����������λ��Ч����)��
(4)Ϊ���ٸ������ø�������CO2�����ڳ�ʼȼ������������________��
(5)�Է�Ӧ�������ɵ�CaSΪԭ�ϣ���һ�������¾�ԭ��������100%�ĸ��·�Ӧ��������CaSO4���÷�Ӧ�Ļ�ѧ����ʽΪ______________________________________����һ�������£�CO2����Զ��ױ���Ӧ�����䱽��������һ���Ȼ�������Ľṹ��ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪���з�Ӧ���Ȼ�ѧ����ʽ��6C(s)��5H2(g)��3N2(g)��9O2(g)===2C3H5(ONO2)3(l)����H1
2H2(g)�� O2(g)===2H2O(g)����H2
C(s)�� O2(g)===CO2(g)����H3
��Ӧ4C3H5(ONO2)3(l)===12CO2(g)��10H2O(g) �� O2(g) ��6N2(g)�Ħ�HΪ(����)
A��12��H3��5��H2��2��H1
B��2��H1��5��H2��12��H3
C��12��H3��5��H2��2��H1
D����H1��5��H2��12��H3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�˵����ȷ����(����)
A�����ں�������ϸ���һЩͭ�飬����Լ���������ǵĸ�ʴ
B��2NO(g)��2CO(g)===N2(g)��2CO2(g)�ڳ��������Է����У���÷�Ӧ�Ħ�H>0
C������0.1 mol��L��1Na2CO3��Һ��CO ��ˮ��̶Ⱥ���Һ��pH������
��ˮ��̶Ⱥ���Һ��pH������
D�������������Ҵ���������Ӧ(��H<0)����������Ũ���Ტ���ȣ��÷�Ӧ�ķ�Ӧ���ʺ�ƽ�ⳣ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ת������Դ���úͻ�����������Ҫ�о����⡣�������������ж��ַ�����
(1)���ռ�����H2S�����Һ���뵽��ͼ��ʾ�ĵ��ص����������е�⡣���������������������·�Ӧ��
S2����2e��===S��(n��1)S��S2��===S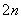
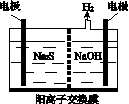
��д�����ʱ�����ĵ缫��Ӧʽ��________________��
�ڵ�������������Һ��ϡ�����ữ�õ����ʣ������ӷ���ʽ��д��__________________________��
(2)��H2S�Ϳ����Ļ������ͨ��FeCl3��FeCl2��CuCl2�Ļ����Һ�з�Ӧ����S��������ת����ͼ��ʾ��
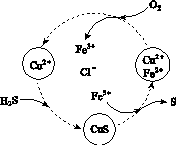
����ͼʾ��ת���У����ϼ۲����Ԫ����________��
�ڷ�Ӧ�е���1 mol H2Sת��Ϊ����ʱ��������Һ��Fe3�������ʵ������䣬������O2�����ʵ���Ϊ________��
�����¶�һ���Ͳ�������Һ�������£�����ͨ�������壬����ֽ��衣��ʹ���ɵ������в���CuS���ɲ�ȡ�Ĵ�ʩ��________________��
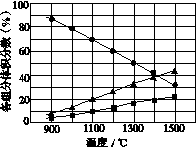
(3)H2S�ڸ����·ֽ�������������H2������Ӧ�ڲ�ͬ�¶��´ﵽƽ��ʱ����������и���ֵ����������ͼ��ʾ��H2S�ڸ����·ֽⷴӦ�Ļ�ѧ����ʽΪ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ں����þ��Ϻ�DZˮͧ����Ϊ��������Դ�������� ��
A��Na2O2 B��NaHCO3 C��SO3(��) D��KMnO4
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com